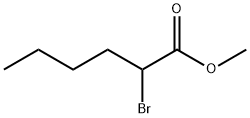
Methyl 2-bromohexanoate synthesis
- Product Name:Methyl 2-bromohexanoate
- CAS Number:5445-19-2
- Molecular formula:C7H13BrO2
- Molecular Weight:209.08

67-56-1
790 suppliers
$9.00/25ml

66-25-1
335 suppliers
$16.80/100G

5445-19-2
253 suppliers
$16.00/25g
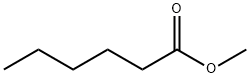
106-70-7
260 suppliers
$14.00/5g
Yield:5445-19-2 80% ,106-70-7 10 %Chromat.
Reaction Conditions:
Stage #1: hexanalwith ammonium cerium (IV) nitrate;lithium bromide in neat liquid;
Stage #2: methanol in neat liquid at 35 - 40; for 3.5 h;
Steps:
Table 2. Oxidation of aldehydes 1a-f with CAN/LiBr to methyl 2-bromocarboxylate 2a-f.
General procedure: To a pear-shaped two-necked reactor (25 mL) equipped with a thermometer and reflux condenser, CAN (2.2 g, 4.0 mmol) and LiBr (261 mg, 3.0 mmol) were added and stirred. Next, aldehyde 1a-f (1 mmol) was added and the reaction mixture stirred for 10-15 min. Then MeOH (64 mg, 2.0 mmol) was added and the reaction mixture stirred for 20 h at 20 °C (3.5 h at 35-40 °C). The reaction mixture was cooled, diluted with water (10-15 mL) and extracted with Et2O (3 * 10-15 mL). The combined organic phases were washed with aqueous NaHCO3 (10 mL) and water (10 mL), then dried (MgSO4). The solvent was removed under reduced pressure (water bath temperature 25-28 °C). The conversion of 1a-f and the yields of 2a-f and 3a-f were determined by GLC using methyl pentanoate and methyl decanoate as internal standards. The isolation of 2-bromoesters 2a-f was performed using gradient silica gel column chromatography with petroleum ether (b.p. 40-70 °C): chloroform (10-1:1), as eluent. Preparative yields of 2a-f were 53-82%
References:
Nikishin, Gennady I.;Kapustina, Nadezhda I.;Sokova, Lyubov L.;Bityukov, Oleg V.;Terent'ev, Alexander O. [Tetrahedron Letters,2017,vol. 58,# 4,p. 352 - 354] Location in patent:supporting information
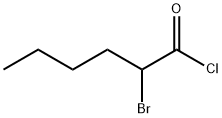
42768-46-7
11 suppliers
inquiry

5445-19-2
253 suppliers
$16.00/25g
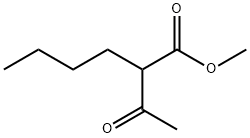
32798-42-8
0 suppliers
inquiry

5445-19-2
253 suppliers
$16.00/25g

106-70-7
260 suppliers
$14.00/5g

5445-19-2
253 suppliers
$16.00/25g

67-56-1
790 suppliers
$9.00/25ml
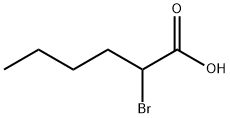
616-05-7
174 suppliers
$21.00/25g

5445-19-2
253 suppliers
$16.00/25g