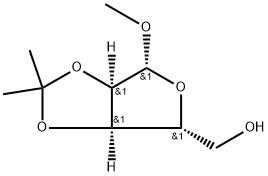
Methyl-2,3-O-isopropylidene-beta-D-ribofuranoside synthesis
- Product Name:Methyl-2,3-O-isopropylidene-beta-D-ribofuranoside
- CAS Number:4099-85-8
- Molecular formula:C9H16O5
- Molecular Weight:204.22

67-56-1
532-20-7

67-64-1

4099-85-8
D-ribose (1; 20.0 g, 133.2 mmol) was suspended in a solvent mixture of acetone (100 mL) and methanol (100 mL) at room temperature. Concentrated sulfuric acid (10 mL) was slowly added and the reaction mixture was stirred for 48 hours. The reaction process was monitored by thin layer chromatography (TLC). Upon completion of the reaction, solid sodium bicarbonate (NaHCO3) was added to neutralize the reaction solution. The insoluble solids were removed by filtration and the filtrate was subsequently concentrated. The concentrated mixture was extracted with ethyl acetate (EtOAc). The organic layers were combined, dried with anhydrous magnesium sulfate (MgSO4), filtered and the solvent was evaporated to remove the organic solvent. The crude product was purified by silica gel fast column chromatography using hexane/ethyl acetate (5:1, v/v) as eluent to afford the target compound ((3aR,4R,6R,6aR)-6-methoxy-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxin-4-yl)methanol as a light yellow oil in 24.4 g (90% yield). The Rf value of the product was 0.2 (eluent: hexane/EtOAc = 5:1, v/v). The specific optical rotation [α]D25 was -80.00 (c 1.00, CHCl3), which is in accordance with the literature value [α]D20 -75.00 (c 1.00, CHCl3).1H NMR (CDCl3, 400 MHz) data were as follows: δ= 4.97 (s, 1H), 4.83 (d, J = 5.7Hz, 1H), 4.58 (d, J = 6.0 Hz, 1H), 4.42 (t, J = 2.7 Hz, 1H), 3.56-3.72 (m, 2H), 3.43 (s, 3H), 3.32 (dd, J = 10.0,3.0 Hz, 1H), 1.49 (s, 3H), 1.32 (s, 3H).

67-56-1
790 suppliers
$9.00/25ml
10257-32-6
6 suppliers
inquiry

67-64-1
0 suppliers
$19.10/10ml

4099-85-8
139 suppliers
$45.00/5G
Yield:4099-85-8 85%
Reaction Conditions:
with sulfuric acid at 5 - 20; for 0.416667 h;Inert atmosphere;Sonication;Time;
Steps:
Synthesis of methyl 2.3-O-isopropylidene-b-D-ribofuranoside (8)
A solution of D-ribose (700 mg, 4.663 mmol) in 5mL of dry acetone and5mL of dry methanol was prepared in a 125mL Erlenmeyer. The mixturewas stirred until the complete solubilization of the sugar. The flask wasthen placed in an ice bath, controlling the temperature under 5 C, whilethe atmosphere was kept dry with nitrogen gas. Subsequently, 0.5mL of98% H2SO4 was added dropwise and slowly, and the mixture was set intoan ultrasound bath. The reaction was allowed to proceed for different times(15, 20, 25, 30, 45, and 60 min). After completion of the reaction, solidNaHCO3 was added until the mixture reached pH 7. The mixture was vacuumfiltrated, and the solvent was evaporated under reduced pressure. Theresulting material was dissolved in 50mL of ethyl acetate and the organiclayer was washed with water (350 mL). The organic layer was dried withanhydrous Na2SO4, filtered, and concentrated under reduced pressure.Product 8 was obtained without further purification as yellow oil in an85% yield. IR (film, CHCl3) m (cm1): 3455.87 (OH), 2940.96 (CH),1457.94, 1378.88 (methyl isopropyl group). 1H NMR (CDCl3, 400.13 MHz):d 1.33 (s, 3H, CH3), 1.49 (s, 3H, CH3), 3.28 (d, 1H, J5,OH 8.5 Hz, OH),3.44 (s, 3H, OCH3), 3.60 (ddd, 1H, J 5a,5b 16.2 Hz and J5,4 3.0 Hz, H-5), 3.68-3.71 (m, 1H, H-5), 4.43 (t, 1H, J4,5 3.0 Hz, H-4), 4.59 (d, 1H,J3,2 5.9 Hz, H-3), 4.83 (d, 1H, J2,3 5.9 Hz, H-2), 4.98 (s, 1H, H-1).13NMR (CDCl3, 100.61 MHz): d 24.86 (CH3), 26.51 (CH3), 55.66 (OCH3),64.15 (C-5), 81.64 (C-2), 85.96 (C-3), 88.49 (C-4), 110.13 (C-1), 112.29 (C-7). The data are in agreement with that in the literature.[30]
References:
Evangelista, Tereza Cristina Santos;Aquino, Gabriel Alves Souto de;Donza, Marcio Roberto H.;Leit?o, Rafael Lisboa;Carvalho, Victor Salarolli de;Kaiser, Carlos Roland;Ferreira, Sabrina Baptista [Journal of Carbohydrate Chemistry,2021,vol. 40,# 5,p. 243 - 268]
50-69-1
744 suppliers
$5.00/5g
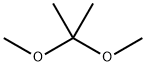
77-76-9
398 suppliers
$9.00/5ml

67-64-1
0 suppliers
$19.10/10ml

4099-85-8
139 suppliers
$45.00/5G

67-56-1
790 suppliers
$9.00/25ml

50-69-1
744 suppliers
$5.00/5g

77-76-9
398 suppliers
$9.00/5ml

4099-85-8
139 suppliers
$45.00/5G

67-56-1
790 suppliers
$9.00/25ml
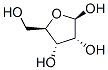
36468-53-8
18 suppliers
inquiry

67-64-1
0 suppliers
$19.10/10ml

4099-85-8
139 suppliers
$45.00/5G