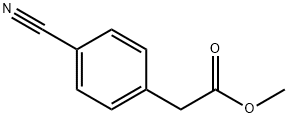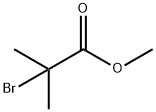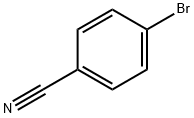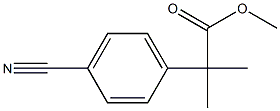
Methyl 2-(4-cyanophenyl)-2-methylpropanoate synthesis
- Product Name:Methyl 2-(4-cyanophenyl)-2-methylpropanoate
- CAS Number:444807-47-0
- Molecular formula:C12H13NO2
- Molecular Weight:203.24
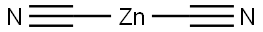
557-21-1
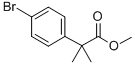
154825-97-5

444807-47-0
Step C: Under argon protection, the intermediate (4.05 g), zinc cyanide (1.3 g) and tetrakis(triphenylphosphine)palladium (456 mg) obtained from step B were dissolved in dry and degassed N,N-dimethylformamide (30 mL), and the reaction was stirred at 80 °C overnight. After completion of the reaction, the mixture was concentrated, diluted with ethyl acetate, washed sequentially with 0.5 N hydrochloric acid and saturated brine, and the organic phase was dried over anhydrous sodium sulfate and purified by silica gel column chromatography (eluent: cyclohexane/ethyl acetate, 9:1 to 4:1) to afford methyl 2-(4-cyanophenyl)-2-methylpropionate (3.05 g, 95% yield) as a colorless oil. Mass spectrometric detection showed [M+H]+ peak of 204.
Yield:444807-47-0 98%
Reaction Conditions:
with sodium hydride in N,N-dimethyl-formamide at 0 - 35; for 2 h;
Steps:
12 Methyl 4-cyano-α,α-dimethylbenzeneacetate
To a solution of methyl 4-cyanobenzeneacetate (2.646 g, 15.1 mmol) and iodomethane (2.4 ml) in N,N-dimethylformamide (50 ml) was slowly added 60% oily sodium hydride (1.55 g, 38.8 mmol) under ice-cooling. The reaction mixture was stirred for 2 hours at room temperature. The reaction solution was slowly added to ice water, and extracted with ethyl acetate. The extracts were washed with a saturated aqueous solution of sodium chloride, dried, and concentrated under reduced pressure. The residue was subjected to a silica gel column chromatography (ethyl acetate/hexane 3:1) to give the title compound (2.996 g, yield 98%). 1H NMR (CDCl3) δ 1.59 (6H, s), 3.67 (3H, s), 7.42-7.47 (2H, m), 7.61-7.65 (2H, m).
References:
EP1541576,2005,A1 Location in patent:Page/Page column 41
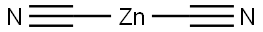
557-21-1
106 suppliers
$12.00/5g

154825-97-5
154 suppliers
$6.00/1g

444807-47-0
28 suppliers
inquiry