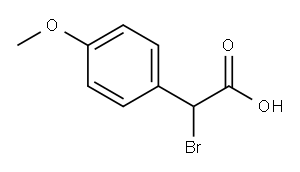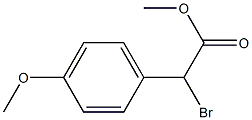
Methyl 2-broMo-2-(4-Methoxyphenyl)acetate synthesis
- Product Name:Methyl 2-broMo-2-(4-Methoxyphenyl)acetate
- CAS Number:50612-99-2
- Molecular formula:C10H11BrO3
- Molecular Weight:259.1
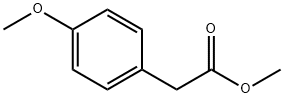
23786-14-3
157 suppliers
$9.00/1g

50612-99-2
30 suppliers
inquiry
Yield:50612-99-2 99%
Reaction Conditions:
with N-Bromosuccinimide;2,2'-azobis(isobutyronitrile) in tetrachloromethane at 80; for 4 h;
Steps:
1.4.5Methyl 2-bromo-2-(4-methoxyphenyl)acetate(18).
NBS (9.06 g, 50.90 mmol) and AIBN (398 mg, 2.43 mmol) was added into a solution 2-(4-methoxyphenyl)acetate17(8.74 g, 48.50 mmol) in carbon tetrachloride (50 ml). The reaction was refluxed at 80°Cfor 4h and quenched with water. Carbon tetrachloride was removed under vacuum. The reaction was extracted with ethyl acetate. The organic phase was washed with sodium thiosulfate (1M) and then saturated sodium chloride, dried over anhydrous sodium sulfate and concentrated to obtain the crude product18(12.43 g, 99% yield) as a white solid.1H NMR (400 MHz, CDCl3) δ 7.52-7.45 (m, 2H), 6.91-6.85 (m, 2H), 5.35 (s, 1H), 3.81 (s, 3H), 3.79 (s, 3H).
References:
Li, Guangzhe;Dong, Huijuan;Ma, Yao;Shao, Kun;Li, Yueqing;Wu, Xiaodan;Wang, Shisheng;Shao;Zhao, Weijie [Bioorganic and Medicinal Chemistry Letters,2019,vol. 29,# 16,p. 2327 - 2331] Location in patent:supporting information
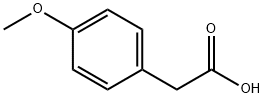
104-01-8
542 suppliers
$6.00/10g

50612-99-2
30 suppliers
inquiry