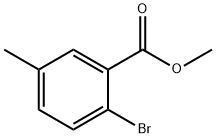
METHYL 2-BROMO-5-METHYLBENZOATE synthesis
- Product Name:METHYL 2-BROMO-5-METHYLBENZOATE
- CAS Number:90971-88-3
- Molecular formula:C9H9BrO2
- Molecular Weight:229.07

67-56-1
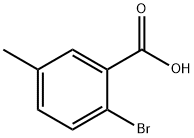
6967-82-4

90971-88-3
Step 1: Preparation of methyl 2-bromo-5-methylbenzoate Oxalyl chloride (0.13 mL, 1.5 mmol) was slowly added to a mixed solution of dichloromethane (DCM, 4 mL) and N,N-dimethylformamide (DMF, 1 drop) of 2-bromo-5-methylbenzoic acid (215 mg, 1.0 mmol) at 0 °C. The reaction mixture was stirred at room temperature for 1 hour before oxalyl chloride (0.13 mL, 1.5 mmol) was added again. After continued stirring for 1 hour, methanol (MeOH, 2 mL) was added and the reaction mixture continued to stir for 6 hours. Upon completion of the reaction, the pH was adjusted to 9 with sodium carbonate (Na2CO3) solution and extracted with dichloromethane (DCM, 2 x 10 mL). The organic layers were combined, washed with saturated saline, dried over anhydrous sodium sulfate (Na2SO4), filtered and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography to afford methyl 2-bromo-5-methylbenzoate (210 mg, 92% yield) as a colorless oil using petroleum ether-ethyl acetate (PE-EA, 15:1) as eluent. 1H NMR (400 MHz, CDCl3): δ 7.60 (d, J = 1.6 Hz, 1H), 7.53 (d, J = 8.8 Hz, 1H), 7.15 (q, J = 3.2 Hz, 1H), 3.92 (s, 3H), 2.33 (s, 3H) ppm.
Yield: 99%
Reaction Conditions:
Stage #1:2-bromo-5-methylbenzoic acid with oxalyl dichloride;N,N-dimethyl-formamide in dichloromethane at 0 - 20; for 1 h;
Stage #2:methanol in dichloromethane at 20; for 3 h;
Steps:
4.3.1 Methyl 2-bromo-5-methylbenzoate (5)8
To a suspension of compound 4 (2.15 g, 10 mmol) in dry DCM (40 mL) and DMF (0.2 mL) at 0 °C was slowly added oxalyl chloride (1.3 mL, 15 mmol). After being stirred for 1 h at room temperature, the reaction mixture was treated with MeOH (20 mL) and then stirred for 3 h. The resulting solution was neutralized with saturated aqueous Na2CO3 (100 mL) and extracted with DCM (70 mL×2). The combined organic layer was washed with water (50 mL) and brine (50 mL), dried over anhydrous Na2SO4. The solvent was removed under reduced pressure and the residue was purified by chromatography on silica gel, eluting with petroleum ether/ethyl acetate (30:1), to afford compound 5 (2.27 g) as colorless oil in 99% isolated yield. 1H NMR (CDCl3, 400 MHz) δ 2.30 (s, 3H), 3.90 (s, 3H), 7.10 (dd, J=2.4, 8.4 Hz, 1H), 7.49 (d, J=8.0 Hz, 1H), 7.57 (d, J=2.0 Hz, 1H); 13C NMR (CDCl3, 100 MHz) δ 20.7, 52.4, 118.2, 131.7, 131.9, 133.5, 134.1, 137.3, 166.8.
References:
Guo, Shenghai;Wang, Jiliang;Li, Yan;Fan, Xuesen [Tetrahedron,2014,vol. 70,# 14,p. 2383 - 2388]

79-37-8
491 suppliers
$17.67/10gm:

6967-82-4
188 suppliers
$6.00/1g

90971-88-3
98 suppliers
$6.00/1g

6967-82-4
188 suppliers
$6.00/1g

90971-88-3
98 suppliers
$6.00/1g
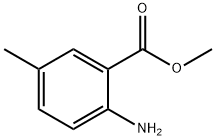
18595-16-9
112 suppliers
$8.00/1g

90971-88-3
98 suppliers
$6.00/1g

6967-82-4
188 suppliers
$6.00/1g

18107-18-1
210 suppliers
$33.00/5g

90971-88-3
98 suppliers
$6.00/1g