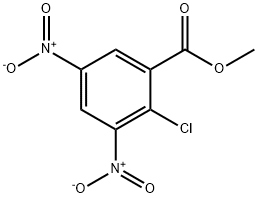
METHYL 2-CHLORO-3,5-DINITROBENZOATE synthesis
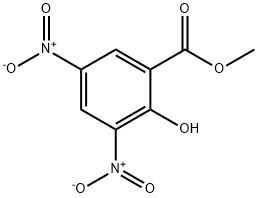
- Product Name:METHYL 2-CHLORO-3,5-DINITROBENZOATE
- CAS Number:2213-79-8
- Molecular formula:C8H5ClN2O6
- Molecular Weight:260.59

67-56-1
790 suppliers
$9.00/25ml
2497-91-8
131 suppliers
$23.89/25g

2213-79-8
12 suppliers
$28.60/1g
Yield:2213-79-8 82%
Reaction Conditions:
with sulfuric acid for 12 h;Reflux;
Steps:
3. Synthesis of PR-104A and corresponding analogs
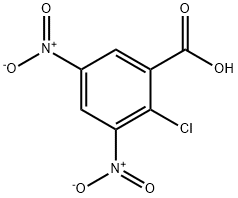
3.1. Methyl 2-chloro-3,5-dinitrobenzoate (2): To a solution of 2-chloro-3,5-dinitrobenzoic acid (20 mmol, 4.93 g) in methanol (35 mL) was added concentrated H2SO4 (0.5 mL). The mixture was refluxed for 12 hours. After completion, as evident by TLC, the reaction mixture was cooled down to room temperature and poured over ice. The slurry was extracted with ether (2 × 50 mL) and the organic layer washed with brine, dried over MgSO4 and concentrated down to furnish 2, as a pale-yellow solid (4.39 g, 82 %). 1H-NMR (500 MHz, CDCl3): 8.81 (d, J=3.3 Hz, 1 H), 8.68 (d, J=3.3 Hz, 1 H), 4.04 (s, 3 H).
References:
Basiri, Alireza;Zhang, Wenting;Garrison, Jered [Bioorganic and Medicinal Chemistry Letters,2021,vol. 31,art. no. 127697] Location in patent:supporting information