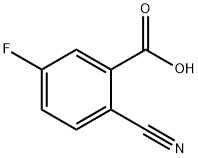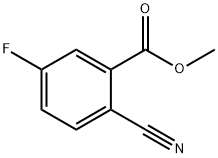
Methyl 2-cyano-5-fluorobenzoate synthesis
- Product Name:Methyl 2-cyano-5-fluorobenzoate
- CAS Number:606080-43-7
- Molecular formula:C9H6FNO2
- Molecular Weight:179.15
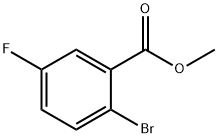
6942-39-8
544-92-3

606080-43-7
(b) Synthesis of methyl 2-cyano-5-fluorobenzoate; Methyl 2-bromo-5-fluorobenzoate (3.0 g, 12.87 mmol; see step (a) above) was dissolved in anhydrous DMF (18 mL). Subsequently, degassing was carried out by passing N2 gas into the solution for 5 min. The reaction mixture was again degassed by adding cuprous(I) cyanide (2.3 g, 25.74 mmol) and then reacted under reflux conditions for 90 min. After completion of the reaction, 10% aqueous NaCN was added and the mixture was extracted with dichloromethane (DCM). The DCM phase was dried by a phase separator and the solvent was subsequently removed under vacuum. The crude product was dissolved in toluene and washed once with deionized water. The organic phase was dried with anhydrous magnesium sulfate (MgSO4) and filtered. Finally, the solvent was removed under vacuum to afford the crude methyl 2-cyano-5-fluorobenzoate (77% yield), which could be used in subsequent steps without further purification.1H NMR (500 MHz, CDCl3) δ 4.04 (s, 3H), 7.38 (dt, 1H), 7.82-7.87 (m, 2H).
Yield:606080-43-7 86%
Reaction Conditions:
with acetyl chloride at 0;
Steps:
21.a
Example 21a) Synthesis of 2-Cyano-5-fluoro-benzoic acid methyl ester (21 a).To a stirred suspension of 16,0 g (96,9 mmol) 2-Cyano-5-fluoro-benzoic acid (Apollo) and 161 ml methanol were added 30.4 g (387,6 mmol) acetyl chloride drop wisely at 00C. The reaction mixture was stirred over night, filtered and concentrated. The residue was diluted with dichloromethane, washed with diluted sodium hydrogen carbonate solution, dried with sodium sulphate and concentrated. The residue was purified by column chromatography (hexane : ethylacetate). The desired product 21a was obtained in 86.0 % yield (14,9 g; 83.3 mmol)MS-ESI: 180 (M+ +1 ,100),Elementary analysis:Calculated: C 60.34% H 3. 38% F 10.60% N 7.82%Determined: C 60.41% H 3, 39% F 10.58% N 7,79%
References:
WO2008/28688,2008,A2 Location in patent:Page/Page column 134