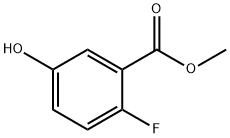
Methyl 2-fluoro-5-hydroxybenzoate synthesis
- Product Name:Methyl 2-fluoro-5-hydroxybenzoate
- CAS Number:1084801-91-1
- Molecular formula:C8H7FO3
- Molecular Weight:170.14

67-56-1
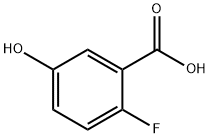
51446-30-1

1084801-91-1
General procedure for the synthesis of methyl 2-fluoro-5-hydroxybenzoate from methanol and 2-fluoro-5-hydroxybenzoic acid: 2-fluoro-5-hydroxybenzoic acid (3e) (780 mg, 5 mmol) was dissolved in 100 mL of methanol. Thionyl chloride (0.8 mL, 10 mmol) was added slowly and dropwise in an ice bath (0 °C) and under stirring conditions, followed by refluxing the reaction mixture for 2 hours. After completion of the reaction, the reaction solution was concentrated and purified by column chromatography (eluent ratio of petroleum ether: ethyl acetate = 2:1) to afford methyl 2-fluoro-5-hydroxybenzoate (3f) 833 mg as a white solid in 98% yield. The product was characterized by 1H NMR (600 MHz, DMSO-d6): δ 9.73 (s, 1H), 7.20-7.18 (m, 1H), 7.13-7.09 (m, 1H), 6.99-6.97 (m, 1H), 3.80 (s, 3H); ESI-MS m/z: calculated value 170.04, measured value 171.25 [M + H]+.

67-56-1
790 suppliers
$7.29/5ml-f

51446-30-1
160 suppliers
$18.00/1g

1084801-91-1
70 suppliers
$30.00/1g
Yield: 90%
Reaction Conditions:
with sulfuric acidReflux;
Steps:
24.B 24B. Methyl 2-fluoro-5-hydroxybenzo
A solution of 2-fluoro-5-hydroxybenzoic acid (1.5 g, 9.6 mmol) and conc. H2SO4 (0.050 mL, 0.92 mmol) in MeOH (10 mL) was stirred at reflux ON. After evaporation of the solvent, the residue was dissolved in 1N NaOH and extracted 3x with EtOAc. The combined extracts were dried over Na2SO4, filtered and evaporated to give 24B (1.47 g, 90% yield) as a colorless solid which was used without further purification in the following reaction.1H NMR (500MHz, CDCl3) δ 7.39 (dd, J=5.5, 3.3 Hz, 1H), 7.08 - 7.03 (m, 1H), 7.03 - 6.98 (m, 1H), 3.95 (s, 3H).
References:
BRISTOL-MYERS SQUIBB COMPANY;SMALLHEER, Joanne M.;KICK, Ellen K.;VALENTE, Meriah Neissel;HU, Carol Hui;HALPERN, Oz Scott;JUSUF, Sutjano WO2018/5336, 2018, A1 Location in patent:Page/Page column 98
127667-01-0
188 suppliers
$10.00/1g

1084801-91-1
70 suppliers
$30.00/1g