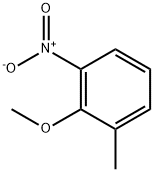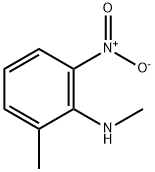
Methyl-(2-Methyl-6-nitro-phenyl)-aMine synthesis
- Product Name:Methyl-(2-Methyl-6-nitro-phenyl)-aMine
- CAS Number:70254-74-9
- Molecular formula:C8H10N2O2
- Molecular Weight:166.18
Yield:70254-74-9 42.1%
Reaction Conditions:
with potassium tert-butylate in N,N-dimethyl-formamide;Heating / reflux;
Steps:
3.1
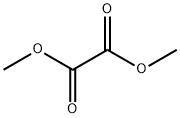
Example 3.1; Methyl-(2-methyl-6-nitro-phenyl)-amine; 2-Methyl-6-nitro-phenylamine (5.0 g, 32.9 mmol), dimethyl oxalate (5.82 g, 49.3 mmol) and potassium tert-butoxide (5.52 g, 49.3 mmol) were dissolved in N,N-dimethylformamide (50 mL). The reaction mixture was kept refluxing for overnight. The reaction was cooled to room temperature; ethyl acetate was then added to the reaction mixture. The reaction mixture was washed with water and brine. The organic phase was separated, dried over anhydrous sodium sulfate, filtered and concentrated in vacuo. The crude residue was purified on silica gel using ethyl acetate:hexane=20%:80% to give product as yellow solid (2.3 g, 42.1%).1H-NMR (300 MHz, CDCl3): (ppm) 2.413 (s, 3H, C-CH3), 3.018 (d, 3H, N-CH3), 7.112 (t, 1H, H-Ar), 7.255 (d, 1H, H-Ar), 7.882 (d, 1H, H-Ar).
References:
US2009/192169,2009,A1 Location in patent:Page/Page column 53
570-24-1
366 suppliers
$6.00/10g

70254-74-9
16 suppliers
inquiry