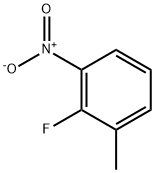
2-Fluoro-3-nitrotoluene synthesis
- Product Name:2-Fluoro-3-nitrotoluene
- CAS Number:437-86-5
- Molecular formula:C7H6FNO2
- Molecular Weight:155.13
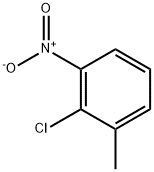
3970-40-9

437-86-5
Cesium fluoride (97.5 g, 642 mmol) was added to a solution of 2-chloro-1-methyl-3-nitrobenzene (73.4 g, 428 mmol) in dimethyl sulfoxide (DMSO, 185 mL) under nitrogen protection. The reaction mixture was stirred at 140 °C for 10 hours. Upon completion of the reaction, the mixture was slowly poured into 0.5 N hydrochloric acid and extracted twice with ethyl acetate. The organic phases were combined, washed with saturated brine and dried over anhydrous magnesium sulfate. The solvent was removed by concentration under reduced pressure to give the crude product. The crude product was purified by reduced pressure distillation (boiling point: 118-122 °C/15 mmHg) to give 2-fluoro-3-nitrotoluene (54.4 g, 82% yield) as a yellow oil.1H NMR (DMSO-d6, 270 MHz) δ (ppm): 7.96 (m, 1H), 7.73 (m, 1H), 7.34 (t, J = 8.2 Hz, 1H), 2.35 (t, J = 8.2 Hz, 1H), 2.35 (t, J = 8.2 Hz, 1H), 2.35 (t, J = 8.2 Hz, 1H). 1H), 2.35 (d, J = 2.4 Hz, 3H). Intersil C18 (ODS, 5 μm, 4.6 mm ID × 50 mm, GL Sciences) or SunFire C18 (ODS, 5 μm, 4.6 mm ID × 50 mm, Waters); mobile phases: water (A) containing 0.05% trifluoroacetic acid and acetonitrile (B) containing 0.05% trifluoroacetic acid; elution method: gradient elution ( Solvent B was raised from 10% to 95% in 3.5 min, then lowered to 10% in 1 min, holding 10% B for 0.5 min); Flow rate: 4.0 mL/min.

3970-40-9
194 suppliers
$8.00/1g

437-86-5
269 suppliers
$7.00/1g
Yield:437-86-5 90%
Reaction Conditions:
with N-benzyl-N,N,N-triethylammonium chloride;sodium fluoride in sulfolane at 130;Reagent/catalyst;Solvent;
Steps:
2; 5; 8 Synthesis of 2-Fluoro-3-nitrotoluene:
2-Chloro-3-nitrotoluene (62.0g, 0.36mol) was dissolved in sulfolane (800mL), sodium fluoride (30.0g, 0.72mol), benzyltriethylammonium chloride (24.0g, 0.11mol) were added ) and polyethylene glycol (18.0g), be warming up to 130 of reaction, drop to room temperature, add water and stir, by steam distillation, obtain 2-fluoro-3-nitrotoluene (50.5g), yield 90%.
References:
CN114560774,2022,A Location in patent:Paragraph 0030-0032; 0039-0041; 0048-0050
95-48-7
459 suppliers
$18.00/25g

437-86-5
269 suppliers
$7.00/1g
13073-29-5
108 suppliers
$8.00/250mg

437-86-5
269 suppliers
$7.00/1g
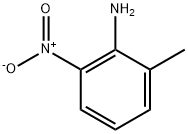
570-24-1
367 suppliers
$6.00/10g

437-86-5
269 suppliers
$7.00/1g
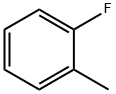
95-52-3
297 suppliers
$10.00/5g
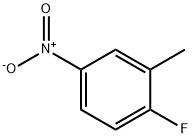
455-88-9
308 suppliers
$6.00/10g
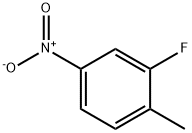
1427-07-2
354 suppliers
$6.00/5g

437-86-5
269 suppliers
$7.00/1g