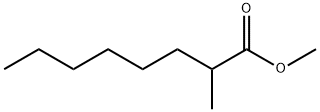
METHYL 2-METHYLOCTANOATE synthesis
- Product Name:METHYL 2-METHYLOCTANOATE
- CAS Number:2177-86-8
- Molecular formula:C10H20O2
- Molecular Weight:172.26

111-66-0
173 suppliers
$10.00/10ml
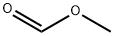
107-31-3
355 suppliers
$17.00/25mL
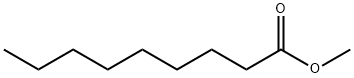
1731-84-6
154 suppliers
$31.00/250mg

2177-86-8
7 suppliers
inquiry
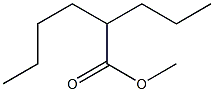
5162-60-7
7 suppliers
inquiry
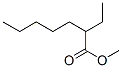
816-63-7
0 suppliers
inquiry
Yield:1731-84-6 92.1 %Chromat.
Reaction Conditions:
with methanesulfonic acid;bis(acetylacetonato)palladium(II);1,2-bis[di(t-butyl)phosphinomethyl]benzene in methanol at 100; under 3825.38 Torr; for 20 h;Autoclave;Inert atmosphere;Overall yield = 98 %Chromat.;Temperature;
Steps:
6
A 100 ml stainless steel autoclave is charged with54.5 mmol of 1 -octene (8.5 ml), Pd(acac)2, 0.038 mol % (6.3 mg), 0.13 mol % of l3uPoX (28.4 mg), 10 ml of methyl formate, 10 ml of methanol and 20 tl of methanesulphonic acid under a protective gas (argon or nitrogen for example). The autoclave is heated to 100° C. to establish a final pressure of 5.1 bar, followed by stirring at that temperature for 20 h. The autoclave is subsequently cooled down to room temperature and the residual pressure is released. A 5 ml quantity of isooctane is added to the reaction solution as an internal standard and the mixture is analysed by gas chromatography. The yield of n-product, i.e. methyl nonanoate, is 92.1%. The yield of branched products (methyl 2-methyloctanoate, methyl 2-ethylheptanoate and methyl 2-propylhexanoate) is altogether 5.9%. The total yield of methyl esters is accordingly 98% with an n:iso ratio of 94:6.
References:
US2014/309435,2014,A1 Location in patent:Paragraph 0042; 0050; 0060

67-56-1
790 suppliers
$9.00/25ml
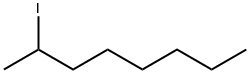
557-36-8
19 suppliers
inquiry
201230-82-2
1 suppliers
inquiry

2177-86-8
7 suppliers
inquiry

67-56-1
790 suppliers
$9.00/25ml
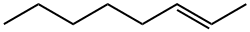
13389-42-9
70 suppliers
$55.00/500mg
201230-82-2
1 suppliers
inquiry

2177-86-8
7 suppliers
inquiry

816-63-7
0 suppliers
inquiry

67-56-1
790 suppliers
$9.00/25ml

111-66-0
173 suppliers
$10.00/10ml
201230-82-2
1 suppliers
inquiry

1731-84-6
154 suppliers
$31.00/250mg

2177-86-8
7 suppliers
inquiry

67-56-1
790 suppliers
$9.00/25ml
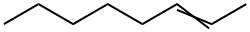
111-67-1
39 suppliers
$28.30/5g

124-38-9
134 suppliers
$214.00/14L

2177-86-8
7 suppliers
inquiry