
methyl 3-bromo-4-oxopentanoate synthesis
- Product Name:methyl 3-bromo-4-oxopentanoate
- CAS Number:95678-51-6
- Molecular formula:C6H9BrO3
- Molecular Weight:209.04
Yield:-
Reaction Conditions:
with bromine in methanol; for 0.416667 h;Heating / reflux;
Steps:
In one embodiment of the present invention, according to Fig. 1, 29.6 g levulinic acid (0.25 mole 98%), dissolved EPO
References:
WO2006/48236,2006,A1 Location in patent:Page/Page column 5-6

75914-07-7
0 suppliers
inquiry

95678-51-6
5 suppliers
inquiry

67-56-1
790 suppliers
$9.00/25ml
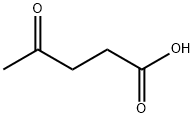
123-76-2
498 suppliers
$5.00/25g
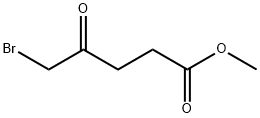
53856-93-2
38 suppliers
inquiry

95678-51-6
5 suppliers
inquiry
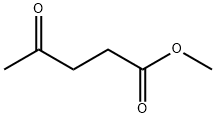
624-45-3
230 suppliers
$10.00/5g

53856-93-2
38 suppliers
inquiry

95678-51-6
5 suppliers
inquiry

140663-07-6
0 suppliers
inquiry