
Methyl 3-fluoro-5-methylbenzoate synthesis
- Product Name:Methyl 3-fluoro-5-methylbenzoate
- CAS Number:660416-38-6
- Molecular formula:C9H9FO2
- Molecular Weight:168.16
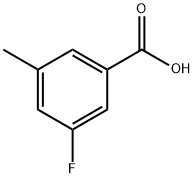
518070-19-4
139 suppliers
$6.00/250mg

74-88-4
356 suppliers
$15.00/10g

660416-38-6
39 suppliers
inquiry
Yield:660416-38-6 100%
Reaction Conditions:
with potassium carbonate in acetone at 65; for 1 h;
Steps:
33.1
Step 1. methyl 3-fluoro-5-methylbenzoateTo a solution of 3-fluoro-5-methylbenzoic acid (1.50 g, 9.73 mmol, Oakwood) inAcetone (40 mL) was added Potassium carbonate (1.34 g, 9.73 mmol) followed by Methyl iodide (0.73 mL, 12 mmol) . The reaction mixture was heated to 65 °C for 1 hour, heating discontinued and stirred overnight, then heating resumed at that temperature for a further 2 hours. Additional Methyl iodide (0.5 mL, 8 mmol) was added and heating was continued for 6 hours. Solids were removed by filtration and acetone was removed in vacuo. The residue was partitioned between IN NaOH and ethyl acetate. The aqueous portion was extracted with a further two portions of ethyl acetate. The combined extracts were washed with brine, then dried over sodium sulfate, decanted and concentrated. The product so obtained was used without further purification (1.64 g, 100%). 1H NMR (300 MHz, CDC13): δ 7.66-7.63 (m, 1H), 7.51 (d, 1H), 7.10-7.04 (m, 1H), 3.91 (s, 3H), 2.40 (s, 3H).
References:
WO2012/68450,2012,A1 Location in patent:Page/Page column 134
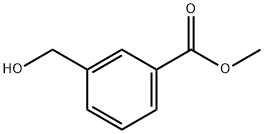
67853-03-6
52 suppliers
$36.00/100mg

660416-38-6
39 suppliers
inquiry

67-56-1
782 suppliers
$9.00/25ml

518070-19-4
139 suppliers
$6.00/250mg

660416-38-6
39 suppliers
inquiry
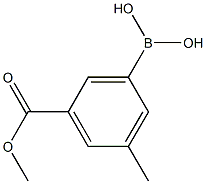
929626-18-6
41 suppliers
$75.00/100mg

660416-38-6
39 suppliers
inquiry
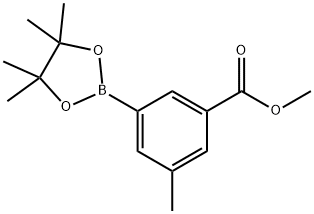
929626-17-5
46 suppliers
$80.00/500mg

660416-38-6
39 suppliers
inquiry