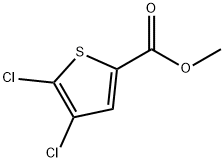
Methyl 4,5-dichlorothiophene-2-carboxylate synthesis
- Product Name:Methyl 4,5-dichlorothiophene-2-carboxylate
- CAS Number:89281-29-8
- Molecular formula:C6H4Cl2O2S
- Molecular Weight:211.07
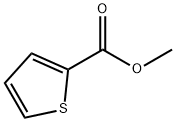
5380-42-7

89281-29-8
The general procedure for the synthesis of methyl 4,5-dichlorothiophene-2-carboxylate from methyl thiophene-2-carboxylate was as follows: methyl thiophene-2-carboxylate (3,142.2 g, 1 mol) was added to a mixed solution of trifluoroacetic acid (TFA, 720 mL) and concentrated sulfuric acid (H2SO4, 180 mL). After cooling the reaction mixture to 0°C, N-chlorosuccinimide (NCS, 333.8 g, 2.5 mol) was added in batches. After addition, the reaction mixture was continued to be stirred for 10 minutes. Subsequently, the reaction solution was quenched by pouring it into cold water. The precipitated solid was collected by filtration, washed with water and dried under vacuum to afford methyl 4,5-dichlorothiophene-2-carboxylate (190 g, 90% yield) as a light yellow solid with a melting point of 58-59°C (literature value 55-56°C). The product was confirmed by infrared spectroscopy (KBr) showing the carbonyl absorption peak located at 1702 cm-1. Nuclear magnetic resonance hydrogen spectrum (1H NMR, CDCl3) showed: δ 7.56 (s, 1H, H-3), 3.89 (s, 3H, -OCH3). Nuclear magnetic resonance carbon spectra (13C NMR, CDCl3) showed: δ 160.8, 132.5, 131.7, 129.6, 125.1, 52.6. Elemental analysis results (C6H4Cl2O2S) Calculated values: C, 34.14; H, 1.91; Measured values: C, 34.01; H, 2.08%. Mass spectrometry (ESI) showed a molecular ion peak m/z 212.1 [M + H]+.

5380-42-7
178 suppliers
$6.00/1g

89281-29-8
39 suppliers
inquiry
Yield: 190 g
Reaction Conditions:
with N-chloro-succinimide;sulfuric acid;trifluoroacetic acid at 0; for 0.166667 h;Time;
Steps:
4,5-Dichlorothiophene-2-carboxylate (4):
Methyl thiophene-2-carboxylate (3, 142.2 g, 1 mol) was added to the mixture of TFA(720 mL) and H2SO4 (180 mL). The solution was cooled to 0 °C andNCS (333.8 g, 2.5 mol) was added in batches. The mixture was stirredfor 10 min and then poured into cold water. The resulting solid wasfiltered, washed with water and dried under vacuum giving methyl4,5-dichlorothiophene-2-carboxylate (190 g, 90%) as pale yellowsolid; m.p. 58-59 °C (lit.22 55-56 °C) IR (KBr): 1702 cm-1 (C=O).1H NMR (CDCl3): 7.56 (s, 1H, H-3), 3.89 (s, 3H, -OCH3). 13C NMR(CDCl3): 160.8, 132.5, 131.7, 129.6, 125.1, 52.6. Anal. calcd forC6H4Cl2O2S: C, 34.14; H, 1.91; found: C, 34.01; H, 2.08%. MS (ESI):m/z 212.1 [M + H]+.
References:
Wang, Peng;Ji, Min;Sha, Fei [Journal of Chemical Research,2014,vol. 38,# 10,p. 622 - 624]

67-56-1
790 suppliers
$7.29/5ml-f
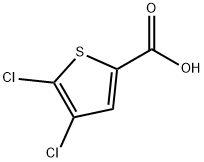
31166-29-7
147 suppliers
$10.00/100mg

89281-29-8
39 suppliers
inquiry
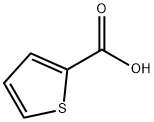
527-72-0
504 suppliers
$5.00/10g

89281-29-8
39 suppliers
inquiry