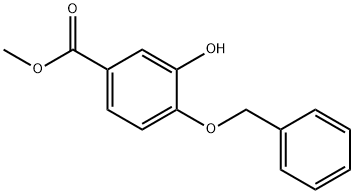
Methyl 4-(benzyloxy)-3-hydroxybenzoate synthesis
- Product Name:Methyl 4-(benzyloxy)-3-hydroxybenzoate
- CAS Number:87687-75-0
- Molecular formula:C15H14O4
- Molecular Weight:258.27
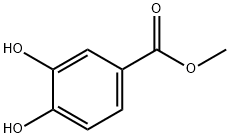
2150-43-8

100-39-0

87687-75-0
Benzyl bromide (0.78 mL, 6.5 mmol, 1.0 eq.) was added slowly and dropwise to a stirred mixed solution of methyl 3,4-dihydroxybenzoate (1.1 g, 6.5 mmol, 1.0 eq.) and potassium carbonate (1.1 g, 7.8 mmol, 1.2 eq.) in N,N-dimethylformamide (11 mL). The reaction mixture was stirred overnight at room temperature. After completion of the reaction, the solvent was removed by rotary evaporator under reduced pressure. The crude product was dissolved in water and extracted with ethyl acetate, followed by adjusting the pH of the aqueous phase with 2 M hydrochloric acid to 2. The organic phase was separated and the aqueous phase was re-extracted with ethyl acetate (3×). The organic phases were combined, dried with anhydrous magnesium sulfate, filtered and concentrated under reduced pressure. The residue was purified by silica gel column chromatography using a gradient elution with 5-80% ethyl acetate/isohexane to afford methyl 4-(benzyloxy)-3-hydroxybenzoate as a white solid (0.82 g, 49% yield). NMR hydrogen spectrum (400 MHz, CDCl3) δ 7.62-7.62 (2H, m), 7.43-7.40 (5H, m), 6.94 (1H, d, J = 8.0 Hz), 5.69 (1H, s), 5.17 (2H, s), 3.88 (3H, s).

2150-43-8
292 suppliers
$13.43/1gm:

100-39-0
438 suppliers
$10.00/10g

87687-75-0
38 suppliers
inquiry
Yield:87687-75-0 78%
Reaction Conditions:
with hydrogen;potassium carbonate in acetonitrile at 80; for 12 h;
Steps:
23.1 Step 1: Methyl 4-benzyloxy-3-hydroxy-benzoate
To a solution of methyl 3, 4-dihydroxybenzoate (10.00 g, 59.47 mmol, 1.00 eq) and potassium carbonate (8.22 g, 59.47 mmol, 1.00 eq) in acetonitrile (120 niL) was added benzyl bromide (10.17 g, 59.47 mmol, 7.06 niL, 1.00 eq). The mixture was stirred at 80 °C for 12 h under hydrogen atmosphere. LCMS showed that the reaction was completed. The mixture was filtered. The filtrate was concentrated under reduced pressure to give the residue. The residue was diluted with water (100 ml.) and extracted with dichloromethane (200 ml,). The organic layer was dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to give a residue. The residue was purified by column chromatography on silica gel (petroleum ether: ethyl acetate = 20: 1 to 10: 1) to give methyl 4-benzyloxy-3 -hydroxy-benzoate (12.00 g, 46.46 mmol, 78% yield) as a white solid. LCMS: MS (ESI) m/z: 259.1[M+1] +. T-I NMR: (400 MHz, CDCb)5:7.69 - 7.57 (m, 2 H), 7.50 - 7.35 (m, 5 H), 6.97 (d, i 8.4 Hz, 1 H), 5.82 - 5.70 (m, 1 H), 5.19 (s, 2 H), 3.94 - 3.84 (m, 3 H). Chemical Formula: C15H14O4, Molecular Weight: 258.27
References:
WO2021/127443,2021,A1 Location in patent:Paragraph 0781-0787

2150-43-8
292 suppliers
$13.43/1gm:

100-51-6
1446 suppliers
$5.00/100g

87687-75-0
38 suppliers
inquiry

2150-43-8
292 suppliers
$13.43/1gm:

100-44-7
658 suppliers
$13.50/250G

87687-75-0
38 suppliers
inquiry
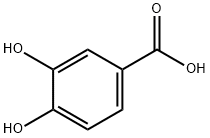
99-50-3
600 suppliers
$10.00/5g

87687-75-0
38 suppliers
inquiry

2150-43-8
292 suppliers
$13.43/1gm:

87687-75-0
38 suppliers
inquiry