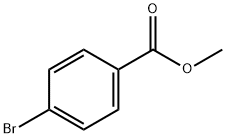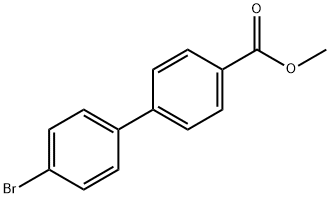
METHYL 4'-BROMO[1,1'-BIPHENYL]-4-CARBOXYLATE synthesis
- Product Name:METHYL 4'-BROMO[1,1'-BIPHENYL]-4-CARBOXYLATE
- CAS Number:89901-03-1
- Molecular formula:C14H11BrO2
- Molecular Weight:291.14
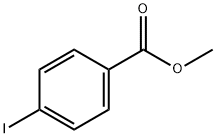
619-44-3
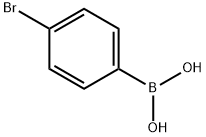
5467-74-3
![METHYL 4'-BROMO[1,1'-BIPHENYL]-4-CARBOXYLATE](/CAS/GIF/89901-03-1.gif)
89901-03-1
Methyl 4-iodobenzoate (9.38 g, 35.8 mmol) and 4-bromophenylboronic acid (7.18 g, 35.8 mmol) were added to Pd(PPh3)4 (2.07 g, 1.79 mmol) in a solvent mixture of toluene (180 mL) and ethanol (100 mL) to form a clarified solution. To this solution was added 4.0 M aqueous sodium carbonate (30 mL). The reaction mixture was refluxed at 80°C for 4 hours. Upon completion of the reaction, the mixture was cooled to room temperature and diluted with ethyl acetate (300 mL). The organic layer was washed sequentially with water (2 x 300 mL), saturated aqueous NaHCO3 (2 x 300 mL) and saturated aqueous NaCl, and then dried over MgSO4. After concentrating the organic phase, the residue was purified by column chromatography (eluent: 7% ethyl acetate-heptane) to afford the white solid product methyl 4'-bromo[1,1'-biphenyl]-4-carboxylate (7.8 g, 78% yield).1H NMR (CDCl3) δ: 8.10 (d, 2H, J = 9.0 Hz), 7.62 (d, 2H, J = 9.0 Hz), 7.59 (d, 2H, J = 9.0 Hz), 7.59 (d, 2H, J = 9.0 Hz), 7.59 (d, 2H, J = 9.0 Hz) 7.59 (d, 2H, J = 9.3 Hz), 7.48 (d, 2H, J = 9.3 Hz), 3.95 (s, 3H).
Yield: 93.62%
Reaction Conditions:
with (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride;potassium carbonate in toluene at 90; for 12 h;Inert atmosphere;
Steps:
12.1 Step 1: 4'-Bromo-[1,1'-biphenyl]-4-carboxylic acid methyl ester
4-bromobenzeneboronic acid (460 mg, 2.3 mmol) was sequentially added to the drying reaction.Methyl 4-iodobenzoate (500 mg, 1.91 mmol),Potassium carbonate (528 mg, 3.82 mmol), (dppf) PdCl2 (140 mg, 0.19 mmol) and toluene (10 mL) were reacted at 90 ° C for 12 h under nitrogen. After the reaction,The reaction solution was filtered, and the filtrate was concentrated under reduced pressure.The residue obtained was subjected to silica gel column chromatography(PE/EtOAc(V/V)=50/1)purification,The title compound is a white solid(520 mg, 93.62%).
References:
Guangdong Dongyangguang Pharmaceutical Co., Ltd.;Ren Qingyun;Liu Xinchang;Huang Jianzhou;Zhang Yingjun;S ·geerdeman CN109111451, 2019, A Location in patent:Paragraph 0556; 0557; 0558; 0559
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
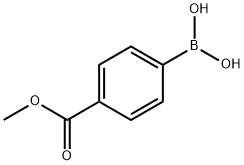
99768-12-4
372 suppliers
$10.00/1 g
![METHYL 4'-BROMO[1,1'-BIPHENYL]-4-CARBOXYLATE](/CAS/GIF/89901-03-1.gif)
89901-03-1
74 suppliers
$53.00/1g

67-56-1
790 suppliers
$7.29/5ml-f
![4'-Bromo-[1,1'-biphenyl]-4-carboxylic acid](/CAS/GIF/5731-11-3.gif)
5731-11-3
67 suppliers
$65.00/250 mg
![METHYL 4'-BROMO[1,1'-BIPHENYL]-4-CARBOXYLATE](/CAS/GIF/89901-03-1.gif)
89901-03-1
74 suppliers
$53.00/1g

619-44-3
318 suppliers
$6.00/10g
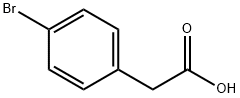
1878-68-8
509 suppliers
$6.00/10g
![METHYL 4'-BROMO[1,1'-BIPHENYL]-4-CARBOXYLATE](/CAS/GIF/89901-03-1.gif)
89901-03-1
74 suppliers
$53.00/1g