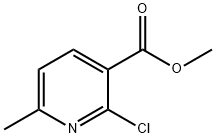
Methyl 4-chloro-6-methylnicotinate synthesis
- Product Name:Methyl 4-chloro-6-methylnicotinate
- CAS Number:53277-47-7
- Molecular formula:C8H8ClNO2
- Molecular Weight:185.61

67-56-1
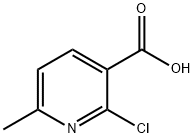
30529-70-5

53277-47-7
The general procedure for the synthesis of methyl 2-chloro-6-methylpyridine-3-carboxylate from methanol and 2-chloro-6-methylnicotinic acid was as follows: a. Oxalyl chloride (9.3 mL) was slowly added to a solution of dichloromethane (500 mL) containing 2-chloro-6-methylnicotinic acid (9 g). The reaction mixture was stirred at room temperature for 30 min before the solvent was removed by concentration under reduced pressure to give the crude product. Subsequently, the crude product was dissolved in methanol (500 mL) and processed at 0 °C with stirring. After the reaction mixture was continued to be stirred at room temperature overnight, the solvent was again removed by concentration under reduced pressure to give the residue. The residue was diluted with water and ethyl acetate (EA), neutralized with saturated aqueous sodium bicarbonate solution and subsequently extracted three times with ethyl acetate. The organic layers were combined, washed with brine, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to give methyl 2-chloro-6-methylpyridine-3-carboxylate (9.7 g, 99% yield). The product was characterized by 1H NMR (400 MHz, chloroform-d): δ 2.60 (s, 3H), 3.95 (s, 3H), 7.17 (d, J = 7.8 Hz, 1H), 8.09 (d, J = 7.8 Hz, 1H).

30529-70-5
295 suppliers
$6.00/1g

74-88-4
356 suppliers
$15.00/10g

53277-47-7
89 suppliers
$20.00/250mg
Yield:53277-47-7 99%
Reaction Conditions:
with potassium carbonate in DMF (N,N-dimethyl-formamide); for 18 h;
Steps:
11; 17
The first step of Scheme 11: To a stirred solution of 2-chloro-6-methyl-nicotinic acid (13.6 g, 80 mmol) in DMF (118 mL) was added potassium carbonate (30 g, 219 mmol) and methyl iodide (23 mL, 366 mmol). After 18 hours, the reaction was diluted with ethyl acetate (300 mL) and washed with water (200 mL). The organic layer was dried over magnesium sulfate and filtered. The solvent was removed under reduced pressure. The material was loaded on silica gel and filtered through a buchner funnel eluding with 1:1 ethyl acetate to yield 14.8 g (99%) of 2-chloro-6-methyl-nicotinic acid methyl ester as a light orange liquid. 1H NMR (400 MHz, CHLOROFORM-D) δ ppm 2.59 (s, 3H) 3.94 (s, 3H) 7.16 (d, J=7.83 Hz, 1H) 8.09 (d, J=7.83 Hz, 1H).
References:
US2005/203081,2005,A1 Location in patent:Page/Page column 12; 30

67-56-1
790 suppliers
$9.00/25ml

30529-70-5
295 suppliers
$6.00/1g

53277-47-7
89 suppliers
$20.00/250mg

30529-70-5
295 suppliers
$6.00/1g

53277-47-7
89 suppliers
$20.00/250mg

30529-70-5
295 suppliers
$6.00/1g

18107-18-1
210 suppliers
$33.00/5g

53277-47-7
89 suppliers
$20.00/250mg

67-56-1
790 suppliers
$9.00/25ml
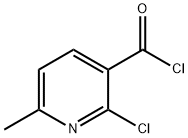
39853-81-1
21 suppliers
$176.72/1gm:

53277-47-7
89 suppliers
$20.00/250mg