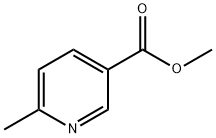
Methyl 6-methylnicotinate synthesis
- Product Name:Methyl 6-methylnicotinate
- CAS Number:5470-70-2
- Molecular formula:C8H9NO2
- Molecular Weight:151.16

67-56-1
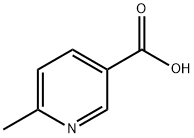
3222-47-7

5470-70-2
To a stirred solution of 6-methylpyridine-3-carboxylic acid (40 g; 290 mmol; 1 equiv.) in methanol (0.75 L) was slowly added concentrated sulfuric acid (40 mL), and the reaction mixture was heated to reflux and held for 17 hours. Upon completion of the reaction, the mixture was concentrated to dryness under reduced pressure. Subsequently, the pH of the residue was adjusted to 7 with ice-cold saturated aqueous NaHCO3 and solid NaHCO3. The aqueous layer was extracted with ethyl acetate (3 x 500 mL), the organic layers were combined, washed with saturated brine, dried with anhydrous sodium sulfate, and the solvent was removed by filtration and under reduced pressure to give methyl 6-methylnicotinate (33 g, 75% yield) as an off-white solid. The product was confirmed by 1H NMR (CDCl3): δ 9.06 (s, 1H), 8.13 (dd, 1H, J = 2,8Hz), 7.20 (d, 1H, J = 8Hz), 3.89 (s, 3H), 2.58 (s, 3H).LCMS analysis showed: m/z = 152.4 [M + H]+, retention time RT = 2.36 min (using program P1, column W).
Yield:5470-70-2 75%
Reaction Conditions:
with sulfuric acid for 17 h;Reflux;
Steps:
3.1 [0153] Step 1: 6-Methyl-nicotinic acid methyl ester
[0154] To a stirred solution of compound I (40 g; 290 mmol; 1 eq) in methanol (0.75 L) was added sulfuric acid (40 mL) and the resulting mixture was heated at reflux for 17 h. The mixture was then evaporated to dryness and the pH was adjusted to 7 using saturated ice-cold aqueous NaHCO3 solution and solid NaHCO3. The organic components were extracted from the aqueous layer with ethyl acetate (3 x 500 mL) and the combined organic layers were washed with brine, dried over anhydrous sodium sulfate, filtered and the solvent was removed in vacuo to afford the title compound (33 g, 75%) as an off-white solid. 1H NMR (CDC13) ? 9.06 (s, 1H), 8.13 (dd, 1H, J = 2, 8 Hz), 7.20 (d, 1H, J = 8 Hz), 3.89 (s, 3H), 2.58 (s, 3H). LCMS: m/z = 152.4 [M+Hj , RT = 2.36 minutes, (Program P1, Column W).
References:
WO2015/95128,2015,A1 Location in patent:Paragraph 0152; 0153; 0154

49668-89-5
6 suppliers
$176.72/250mgs:

5470-70-2
537 suppliers
$5.00/5g

3222-47-7
358 suppliers
$6.00/1g

144-55-8
1411 suppliers
$10.00/25g

5470-70-2
537 suppliers
$5.00/5g

3222-47-7
358 suppliers
$6.00/1g

5470-70-2
537 suppliers
$5.00/5g
1121-78-4
286 suppliers
$6.00/5g

5470-70-2
537 suppliers
$5.00/5g