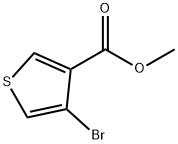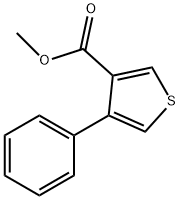
METHYL 4-PHENYLTHIOPHENE-3-CARBOXYLATE synthesis
- Product Name:METHYL 4-PHENYLTHIOPHENE-3-CARBOXYLATE
- CAS Number:38695-71-5
- Molecular formula:C12H10O2S
- Molecular Weight:218.27
Yield:38695-71-5 100%
Reaction Conditions:
with tetrakis(triphenylphosphine) palladium(0);sodium carbonate in 1,4-dioxane;water at 90; for 5 h;Suzuki-Miyaura Coupling;
Steps:
2 4.1.2. Methyl 4-phenylthiophene-3-carboxylate (6)
General procedure: 4.1.1
Methyl 3-phenylthiophene-2-carboxylate (5)
Tetrakis(triphenylphosphine)palladium (5.49 g, 4.75 mmol) and 2 M aqueous disodium carbonate (71 mL) were added to a mixture of methyl 3-bromothiophene-2-carboxylate (1, 21 g, 95.0 mmol), phenylboronic acid (13.9 g, 114 mmol) and 1,4-dioxane (210 mL), and the mixture was stirred for 5 h at 90 °C.
The reaction mixture was cooled to room temperature and then diluted with H2O and extracted with AcOEt.
The organic layer was washed successively with H2O and saturated aqueous NaCl and then dried and concentrated in vacuo.
The residue was chromatographed on silica gel with elution using hexane/AcOEt (9:1 to 4:1) to produce the desired compound 5 (14.4 g, 70%) as a colorless solid which was used in the next reaction without recrystallization. 4.1.2
Methyl 4-phenylthiophene-3-carboxylate (6)
Compound 6 was prepared from methyl 4-bromothiophene-3-carboxylate 2 in quantitative yield as a colorless oil, using an approach similar to that described for 5, and was used in the next reaction without further purification. 1H NMR (DMSO-d6) δ 3.68 (3H, s), 7.32-7.39 (5H, m), 7.56 (1H, d, J = 3.2 Hz), 8.37 (1H, d, J = 3.2 Hz); FAB MS m/e [M+Na]+ 241.
References:
Nagashima, Shinya;Matsushima, Yuji;Hamaguchi, Hisao;Nagata, Hiroshi;Kontani, Toru;Moritomo, Ayako;Koshika, Tadatsura;Takeuchi, Makoto [Bioorganic and Medicinal Chemistry,2014,vol. 22,# 13,p. 3478 - 3487]
2462-02-4
25 suppliers
inquiry

38695-71-5
18 suppliers
inquiry
532-27-4
192 suppliers
$9.00/1g

38695-71-5
18 suppliers
inquiry