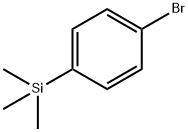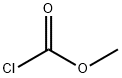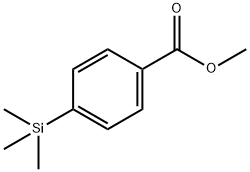
Methyl 4-(Trimethylsilyl)benzoate synthesis
- Product Name:Methyl 4-(Trimethylsilyl)benzoate
- CAS Number:22515-30-6
- Molecular formula:C11H16O2Si
- Molecular Weight:208.33

24424-99-5
859 suppliers
$13.50/25G
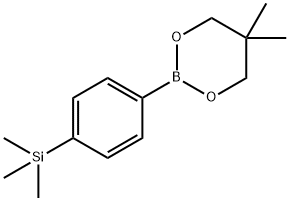
574755-28-5
1 suppliers
inquiry

74-88-4
358 suppliers
$15.00/10g

22515-30-6
20 suppliers
inquiry
Yield:22515-30-6 92%
Reaction Conditions:
Stage #1: di-tert-butyl dicarbonate;(4-(5,5-dimethyl-1,3,2-dioxaborinan-2-yl)phenyl)trimethylsilanewith 4,4'-dimethyl-2,2'-bipyridines;lithium methanolate;copper(l) chloride in N,N-dimethyl acetamide at 30; for 6 h;Inert atmosphere;Schlenk technique;
Stage #2: methyl iodide in N,N-dimethyl acetamide at 30; for 2 h;Schlenk technique;
Steps:
Methyl (Het)arylcarboxylates 3a-y, 5a-e; General Procedure
General procedure: A 15 mL Schlenk tube equipped with a stirrer bar was chargedwith CuCl (10 mol%), L7 (13 mol%), LiOMe (2.5 equiv), and theappropriate boronic ester 1 or 4 (0.375 mmol). The vessel wasthen evacuated and filled with Ar (three cycles). DMA (0.5 mL)and (Boc)2O (0.25 mmol) were added sequentially under Ar, andthe mixture was stirred at 30 for 6 h. MeI (5 equiv) was thenadded in air, and the mixture was stirred at 30 for additional2 h. The mixture was finally diluted with EtOAc and washedwith sat. aq NaCl (20 mL). The aqueous phase was furtherextracted with EtOAc (3 × 20 mL), and the combined organicphases were dried (Na2SO4) and concentrated. The residue waspurified by column chromatography [silica gel EtOAc-hexane(1:100 to 1:50)].
References:
Xu, Jin-Di;Su, Xiao-Bo;Wang, Cai;Yao, Li-Wei;Liu, Jing-Hui;Hu, Guo-Qin [Synlett,2021,vol. 32,# 8,art. no. ST-2020-U0651-L,p. 833 - 837] Location in patent:supporting information
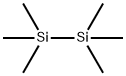
1450-14-2
286 suppliers
$29.12/10ML
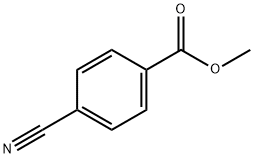
1129-35-7
301 suppliers
$8.00/5g

22515-30-6
20 suppliers
inquiry
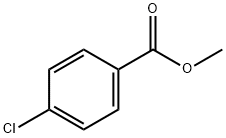
1126-46-1
237 suppliers
$8.00/25g

1450-14-2
286 suppliers
$29.12/10ML

22515-30-6
20 suppliers
inquiry
![Benzoic acid, 4-[(trimethylsilyl)carbonyl]-, methyl ester](/CAS/20210305/GIF/75748-12-8.gif)
75748-12-8
0 suppliers
inquiry

22515-30-6
20 suppliers
inquiry