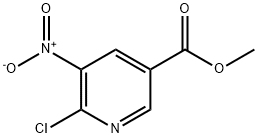
Methyl-6-chloro-5-nitronicotinate synthesis
- Product Name:Methyl-6-chloro-5-nitronicotinate
- CAS Number:59237-53-5
- Molecular formula:C7H5ClN2O4
- Molecular Weight:216.58

67-56-1
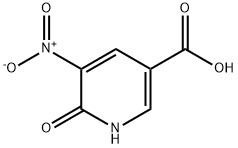
6635-31-0

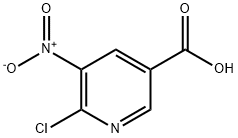
59237-53-5
The general procedure for the synthesis of methyl 6-chloro-5-nitronicotinate from methanol and 5-nitro-6-hydroxynicotinic acid was as follows: DMF (0.15 eq.) was added to a solution of 5-nitro-6-hydroxynicotinic acid (1 eq.) in SOCl2 (4.7 eq.). The reaction mixture was heated to reflux for 8 hours followed by vacuum concentration. The concentrated residue was dissolved in CH2Cl2 and the solution was cooled to -40°C. Methanol (1.4 eq.) was slowly added while keeping the internal temperature below -30°C. Subsequently, an aqueous NaHCO3 solution (1 eq.) was added and the mixture was gradually warmed to room temperature. The organic phase was separated and concentrated under vacuum. Finally, the crude product was purified by crystallization from ethanol to afford methyl 6-chloro-5-nitronicotinate in 90% yield.

67-56-1
790 suppliers
$7.29/5ml-f

6635-31-0
234 suppliers
$8.00/1g

59237-53-5
178 suppliers
$11.00/250mg
Yield:59237-53-5 90%
Reaction Conditions:
Stage #1:6-hydroxy-5-nitronicotinic acid with thionyl chloride;N,N-dimethyl-formamide for 8 h;Reflux;
Stage #2:methanol in dichloromethane at -40 - -30;
Stage #3: with sodium hydrogencarbonate in dichloromethane;water at -30 - 20;
Steps:
1
DMF (0.15 eq) was added to a solution of β-hydroxy-S-nitronicotinic acid (1 eq) in SOCl2 (4.7 eq). The mixture was heated at reflux for 8 h then concentrated in vacuo. The residue was taken up in CH2Cl2, cooled to -400C, and MeOH (1.4 eq) added while maintaining the internal temperature below -300C. Aqueous NaHCO3 (1 eq) was added and the mixture allowed to warm to room temperature. The organic phase was separated and concentrated in vacuo. The crude residue was crystallized from EtOH to give methyl 6-chloro-5-nitronicotinate (90 % yield)
References:
SIRTRIS PHARMACEUTICALS, INC.;OALMANN, Christopher;DISCH, Jeremy, S.;NG, Pui, Yee;PERNI, Robert, B. WO2010/71853, 2010, A1 Location in patent:Page/Page column 68
7477-10-3
118 suppliers
$6.00/250mg

74-88-4
356 suppliers
$15.00/10g

59237-53-5
178 suppliers
$11.00/250mg

67-56-1
790 suppliers
$7.29/5ml-f

59237-53-5
178 suppliers
$11.00/250mg

7477-10-3
118 suppliers
$6.00/250mg
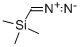
18107-18-1
210 suppliers
$33.00/5g

59237-53-5
178 suppliers
$11.00/250mg

67-56-1
790 suppliers
$7.29/5ml-f

7477-10-3
118 suppliers
$6.00/250mg

59237-53-5
178 suppliers
$11.00/250mg