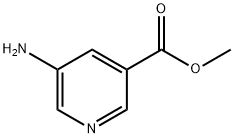
Methyl 5-aminopyridine-3-carboxylate synthesis
- Product Name:Methyl 5-aminopyridine-3-carboxylate
- CAS Number:36052-25-2
- Molecular formula:C7H8N2O2
- Molecular Weight:152.15

67-56-1
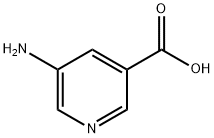
24242-19-1

36052-25-2
SOCl2 (10.4 g, 86.9 mmol) was slowly added dropwise to a stirred methanolic solution (100 mL) of 5-aminonicotinic acid (10.0 g, 72.5 mmol) at 0 °C. After gradually warming the reaction mixture to room temperature, the reaction was continued at reflux for 16 hours. After completion of the reaction, the mixture was cooled and concentrated under vacuum. The residue was diluted with deionized water (200 mL) and subsequently neutralized with aqueous NaHCO3 to pH=7. The aqueous phase mixture was extracted with dichloromethane (DCM, 100 mL x 2). The organic layers were combined, washed with saturated saline (100 mL × 2), dried over anhydrous Na2SO4, filtered, and the filtrate was concentrated to dryness under vacuum to give 9.5 g of methyl 5-aminopyridine-3-carboxylate as a white solid in 86% yield. The product was characterized by 1H NMR (DMSO-d6, 400 MHz): δ=8.24 (1H, d), 8.12 (1H, d), 7.42 (1H, dd), 5.65 (2H, brs), 3.84 (3H, s).

67-56-1
791 suppliers
$7.29/5ml-f

24242-19-1
264 suppliers
$6.00/1g

36052-25-2
135 suppliers
$7.00/250mg
Yield: 86%
Reaction Conditions:
with thionyl chloride at 0;Reflux;
Steps:
IV-16.1
To a stirred solution of 5-amino-nicotinic acid (10.0 g, 72.5 mmol) in methanol (100 mL) was added SOCl2 (10.4 g, 86.9 mmol) dropwise at 0°C. The mixture was allowed warm to room temperature and then refluxed for 16 hours. The mixture was cooled, concentrated in vacuum and the residue was diluted with water (200 mL). The mixture was neutralized with aqueous NaHC03 solution to pH = 7. The aqueous mixture was extracted with DCM (100 mL x2). The combined organic layers were washed with brine (100 mL x2), dried over anhydrous Na2S04, filtered and the filtrate was concentrated in vacuum to dryness to give 9.5 g (yield: 86%) of 5-amino-nicotinic acid methyl ester as white solid. 1H NMR (DMSO-d6, 400MHz): δ = 8.24 (1H, d), 8.12 (1H, d), 7.42 (1H, dd), 5.65 (2H, brs), 3.84 (3H, s).
References:
SANFORD-BURNHAM MEDICAL RESEARCH INSTITUTE;PINKERTON, Anthony, B.;DAHL, Russell;COSFORD, Nicholas, D.P.;MILLAN, Jose, Luis WO2013/126608, 2013, A1 Location in patent:Paragraph 00492-00493

24242-19-1
264 suppliers
$6.00/1g

36052-25-2
135 suppliers
$7.00/250mg

24242-19-1
264 suppliers
$6.00/1g

18107-18-1
210 suppliers
$33.00/5g

36052-25-2
135 suppliers
$7.00/250mg
29681-44-5
297 suppliers
$6.00/5g

36052-25-2
135 suppliers
$7.00/250mg
20826-04-4
559 suppliers
$5.00/1g

36052-25-2
135 suppliers
$7.00/250mg