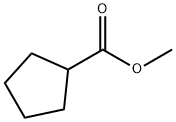
METHYL CYCLOPENTANECARBOXYLATE synthesis
- Product Name:METHYL CYCLOPENTANECARBOXYLATE
- CAS Number:4630-80-2
- Molecular formula:C7H12O2
- Molecular Weight:128.17

67-56-1
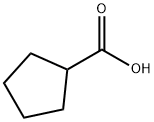
3400-45-1

4630-80-2
Cyclopentanecarboxylic acid (9.5 mL, 87.6 mmol) was dissolved in anhydrous CH2Cl2 (100 mL) in an oven-dried flask equipped with anhydrous CaCl2 drying tube. Subsequently, oxalyl chloride (8.8 mL, 105.1 mmol) was slowly added dropwise and 2 drops of DMF was added as a catalyst. After the reaction was carried out for 7 hours, the mixture was concentrated under vacuum. The concentrated residue was dissolved in MeOH (25 mL) and the reaction was continued for 19 hours. After completion of the reaction, the mixture was again concentrated under vacuum. The residue was redissolved in CH2Cl2 (100 mL) and washed sequentially with saturated NaHCO3 solution and brine. The organic layers were combined, dried with anhydrous Na2SO4, filtered and concentrated under vacuum. Finally, the residue was purified by simple distillation to afford methyl cyclopentanoate (9.5 g, 74.3 mmol, 85% yield) as a colorless oil.

67-56-1
790 suppliers
$7.29/5ml-f

3400-45-1
385 suppliers
$10.00/5g

4630-80-2
202 suppliers
$12.00/1g
Yield:4630-80-2 85%
Reaction Conditions:
Stage #1:cyclopentanecarboxylic acid with oxalyl dichloride;N,N-dimethyl-formamide in dichloromethane at 20; for 7 h;
Stage #2:methanol at 20; for 19 h;
Steps:
Procedure for the preparation of ester 2.8e
To a solution of cyclopentanecarboxylic acid (9.5 mL, 87.6 mmol) in dry CH2Cl2 (100mL) in an oven-dried flask equipped with an anhydrous CaCl2 tube was added oxalylchloride (8.8 mL, 105.1 mmol) dropwise, followed by 2 drops of DMF. After 7 h, theresulting mixture was concentrated in vacuo and the residue was taken up with MeOH(25 mL). After 19 h, the mixture was concentrated in vacuo and the residue was dissolvedin CH2Cl2 (100 mL), then washed with saturated NaHCO3 and brine. The combinedorganic layers were dried over anhydrous Na2SO4, filtered and concentrated in vacuo.The residue was purified by simple distillation to afford 2.8e (9.5 g, 74.3 mmol, 85 %yield) as a colourless oil.
References:
Lo, Kai-Yip;Ye, Liu;Yang, Dan [Synlett,2017,vol. 28,# 13,p. 1570 - 1575] Location in patent:supporting information

67-56-1
790 suppliers
$7.29/5ml-f
201230-82-2
1 suppliers
inquiry
142-29-0
262 suppliers
$11.00/25mL

4630-80-2
202 suppliers
$12.00/1g

67-56-1
790 suppliers
$7.29/5ml-f
201230-82-2
1 suppliers
inquiry

142-29-0
262 suppliers
$11.00/25mL
25662-28-6
135 suppliers
$8.00/1g

4630-80-2
202 suppliers
$12.00/1g

120-92-3
427 suppliers
$11.00/25g
598-99-2
167 suppliers
$21.21/10gm:

3400-45-1
385 suppliers
$10.00/5g

4630-80-2
202 suppliers
$12.00/1g

67-56-1
790 suppliers
$7.29/5ml-f

124-38-9
134 suppliers
$175.00/23402

142-29-0
262 suppliers
$11.00/25mL

4630-80-2
202 suppliers
$12.00/1g