
methyl furo[2,3-b]pyridine-5-carboxylate synthesis
- Product Name:methyl furo[2,3-b]pyridine-5-carboxylate
- CAS Number:169815-80-9
- Molecular formula:C9H7NO3
- Molecular Weight:177.1568
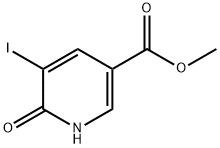
885950-46-9
46 suppliers
$33.00/100mg
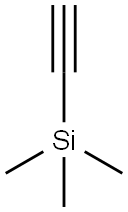
1066-54-2
472 suppliers
$9.00/10g
![methyl furo[2,3-b]pyridine-5-carboxylate](/CAS/20200401/GIF/169815-80-9.gif)
169815-80-9
7 suppliers
inquiry
Yield:169815-80-9 16.2%
Reaction Conditions:
with copper(l) iodide;tetrakis(triphenylphosphine) palladium(0);N-ethyl-N,N-diisopropylamine in tetrahydrofuran at 65;Inert atmosphere;
Steps:
16.16b Step 16b: Preparation of methyl furo[2,3-b]pyridine-5-carboxylate (compound 0206-49):
Under nitrogen protection,6-Hydroxy-5-iodonicotinic acid methyl ester (0204-49) (1.45 g, 5.2 mmol, 1.0 equiv),Trimethylsilylacetylene (2.55 g, 26 mmol, 5.0 equiv),Copper iodide (494 mg, 2.6 mmol, 0.5 equiv) andTetratriphenylphosphine Palladium (168 mg, 0.16 mmol, 0.03 eq)Add to 30 ml of tetrahydrofuran,Diisopropylethylamine (1.3 g, 13 mmol, 2.5 eq.) was then added dropwise.The mixture was heated to 65[deg.] C. and the reaction was stirred overnight.Cool to room temperature, filter,The residue was concentrated under reduced pressure and diluted with 50 ml of anhydrous methanol.Potassium carbonate (1.07 g, 7.8 mmol, 1.5 equiv) was added andCuprous iodide (1.0 g, 5.2 mmol, 1.0 eq).After reacting at 60°C for 4 hours,The mixture was cooled to room temperature, filtered by suction, and the filtrate was concentrated under reduced pressure and purified by silica gel column chromatography (eluent: petroleum ether:ethyl acetate=25:1).The product was obtained as a yellow solid (150 mg, yield: 16.2%).
References:
CN107383024,2017,A Location in patent:Paragraph 0223
5006-66-6
486 suppliers
$13.43/1gm:
![methyl furo[2,3-b]pyridine-5-carboxylate](/CAS/20200401/GIF/169815-80-9.gif)
169815-80-9
7 suppliers
inquiry

885950-46-9
46 suppliers
$33.00/100mg
![methyl furo[2,3-b]pyridine-5-carboxylate](/CAS/20200401/GIF/169815-80-9.gif)
169815-80-9
7 suppliers
inquiry
66171-50-4
171 suppliers
$12.00/1/ G
![methyl furo[2,3-b]pyridine-5-carboxylate](/CAS/20200401/GIF/169815-80-9.gif)
169815-80-9
7 suppliers
inquiry
![FURO[2,3-B]PYRIDINE-5-CARBOXYLIC ACID](/CAS/GIF/122534-94-5.gif)
