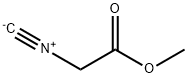
METHYL ISOCYANOACETATE synthesis
- Product Name:METHYL ISOCYANOACETATE
- CAS Number:39687-95-1
- Molecular formula:C4H5NO2
- Molecular Weight:99.09
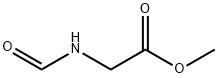
3154-54-9
52 suppliers
$11.00/1g

39687-95-1
149 suppliers
$31.00/1g
Yield:39687-95-1 65%
Reaction Conditions:
Stage #1:methyl N-formylglycinate with triethylamine in dichloromethane for 0.25 h;Cooling with ice;
Stage #2: with trichlorophosphate in dichloromethane for 1 h;Cooling with ice;
Steps:
The synthesis of α-isocyanomethyl acetate is as follows:
2.75 g (23.5 mmol) of N-formyl glycine methyl ester is dissolved in 100 mL of re-distilled anhydrous dichloromethane (DCM), and then triethylamine 8.1 is added mL (58.7 mmol), and the reaction mixture was cooled in an ice bath with stirring for 15 min. After cooling was completed, 2.2 mL (23.5 mmol) of phosphorus oxychloride was slowly added dropwise to the inverted system, and then the reaction was continued in an ice bath with stirring for 1 h. The solution was then warmed to room temperature, and a saturated Na2CO3 solution was slowly added until the pH showed alkaline. Stirring was continued until the aqueous and organic phases were fully separated, the organic layer was separated from the separatory funnel, and the aqueous layer was extracted with DCM (30 mL x 3). The organic layers were combined, washed three times with saturated brine, and the organic phase was dried over anhydrous MgSO4, filtered and washed. The filtrates were combined and spin-dried to obtain the crude α-isocyanoacetate. After drying, 1.51 g of a dark brown liquid with a strong pungent odor was obtained with a yield of 65.0%.
References:
Suzhou Lanyun Pharmaceutical Technology Co., Ltd.;Jin Wei;Liu Jinlun;Wang Jianbing CN110305067, 2019, A Location in patent:Paragraph 0028; 0029
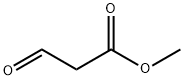
63857-17-0
17 suppliers
inquiry

39687-95-1
149 suppliers
$31.00/1g