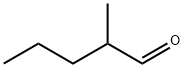
Methyl valeraldehyde synthesis
- Product Name:Methyl valeraldehyde
- CAS Number:123-15-9
- Molecular formula:C6H12O
- Molecular Weight:100.16
201230-82-2
1 suppliers
inquiry

109-68-2
72 suppliers
$41.00/25mL
97-96-1
140 suppliers
$10.00/5mL

123-15-9
174 suppliers
$10.00/5mL
Yield:-
Reaction Conditions:
with tetrakis(2,4-di-tert-butylphenyl)homopiperazine-1,4-diyl bis(phosphonite);hydrogen at 120; under 37503.8 Torr; for 4 h;Autoclave;Inert atmosphere;Overall yield = 79.5 %;Reagent/catalyst;
Steps:
The hydroformylation experiments were conducted in a 200 ml autoclave equipped with a thermocouple, a Bronkhorst HITEC mass flow meter and a Bronkhorst pressure regulator, at 120° C. and a pressure of 50 bar of synthesis gas (99.997%; CO/H2=1:1). The reaction was effected at a constant pressure over a period of four hours. The autoclave together with the storage vessel for the olefin addition was purged repeatedly with argon before the catalyst solution (=metal complex+ligand+solvent) was introduced into the reactor and the olefin to the reservoir vessel (argon countercurrent). In a typical experiment, olefin (15 ml) and catalyst solution (41 ml) were used with an olefin/rhodium ratio of 2000/1. The catalyst solution was heated to the desired reaction temperature under synthesis gas for 30 minutes. After the addition of olefin, the pressure was kept at 50 bar and the gas consumption was measured with a mass flow meter. After four hours, the autoclave was cooled down to room temperature and the pressure was released. The product analysis was effected by gas chromatography; for this purpose, the reaction solution (1 ml) was diluted with n-pentane (10 ml) and toluene was used as internal standard.
References:
US2017/174601,2017,A1 Location in patent:Paragraph 0076; 0077

109-67-1
186 suppliers
$35.00/25g
201230-82-2
1 suppliers
inquiry

123-15-9
174 suppliers
$10.00/5mL

66-25-1
335 suppliers
$16.80/100G

109-66-0
456 suppliers
$10.00/25ml

109-68-2
72 suppliers
$41.00/25mL

123-15-9
174 suppliers
$10.00/5mL

66-25-1
335 suppliers
$16.80/100G
22160-39-0
24 suppliers
inquiry

123-15-9
174 suppliers
$10.00/5mL

109-67-1
186 suppliers
$35.00/25g

124-38-9
134 suppliers
$214.00/14L

123-15-9
174 suppliers
$10.00/5mL

66-25-1
335 suppliers
$16.80/100G