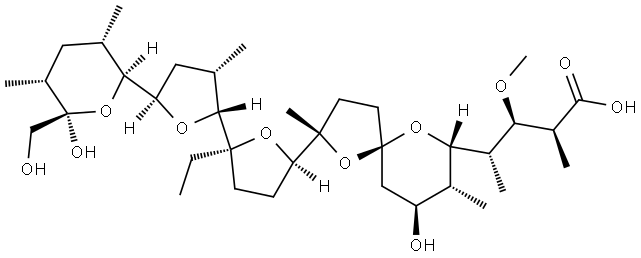
Monensin synthesis
- Product Name:Monensin
- CAS Number:17090-79-8
- Molecular formula:C36H62O11
- Molecular Weight:670.87
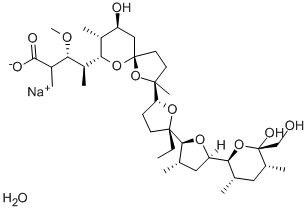
22373-78-0
318 suppliers
$25.00/500mg

17090-79-8
204 suppliers
inquiry
Yield:-
Reaction Conditions:
with sulfuric acid in dichloromethane;water; pH=1.5
Steps:
4.2. Preparation of monensin A amide with AB15C5 amine
Monensin A sodium salt (MON-Na) was dissolved in dichloromethane (CH2Cl2) and stirred vigorously with a layer of aqueous sulfuric acid (H2SO4; pH=1.5). The organic layer containing MONA was washed with distilled water, and dichloromethane was then evaporated under reduced pressure to dryness. A solution of MONA (1000 mg, 1.49 mmol), 1,3-dicyclohexylcarbodiimide (DCC-515 mg, 2.50 mmol) and 4-aminobenzo-15-crown-5 (AB15C5-1133 mg, 4.00 mmol) all dissolved in dichloromethane and 1-hydroxybenzotriazole (HOBt-330 mg, 2.44 mmol) dissolved in tetrahydrofuran was mixed together and stirred at a temperature between 0 and -10 °C for 24 h. After this time the reaction mixture was stirred at room temperature for the next 24 h, diluted with H2O and extracted with CH2Cl2. The organic phase was evaporated under reduced pressure to dryness. The residue was suspended in hexane and filtered off to remove 1,3-dicyclohexylurea (DCU) as a by-product. The filtrate was evaporated under reduced pressure and purified by chromatography on silica gel (Fluka type 60) to give M-AM3 (988 mg, 71% yield) as an amorphous, brown solid.Elemental analysis for M-AM3 (C50H81NO15): Calculated: C 64.15%, H 8.72%, N 1.50%. Found: C 64.05%, H 8.78%, N 1.61%.Furthermore, M-AM3 and its 1:1 and 1:2 complexes with sodium perchlorate were fully characterised by ESIMS, 1H, 13C NMR and FTIR spectroscopy.
References:
Łowicki, Daniel;Huczyński, Adam;Stefańska, Joanna;Brzezinski, Bogumil [Tetrahedron,2011,vol. 67,# 7,p. 1468 - 1478] Location in patent:experimental part