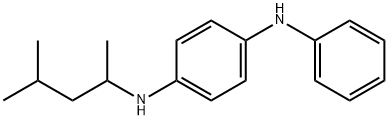
N-(1,3-Dimethylbutyl)-N'-phenyl-p-phenylenediamine synthesis
- Product Name:N-(1,3-Dimethylbutyl)-N'-phenyl-p-phenylenediamine
- CAS Number:793-24-8
- Molecular formula:C18H24N2
- Molecular Weight:268.4
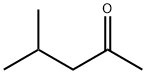
108-10-1
907 suppliers
$12.00/25ml
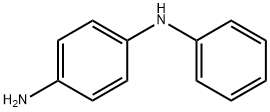
101-54-2
223 suppliers
$14.00/25g

793-24-8
271 suppliers
$5.00/1g
Yield:793-24-8 99.4%
Reaction Conditions:
with hydrogen;pyrographite at 20 - 150; under 22052.2 Torr; for 0.1 h;Autoclave;
Steps:
1 (Production Example 2 of antioxidant from aniline (Method of synthesis of antioxidant 6PPD-1 in Table 1))
Two molecules of the acetone synthesized above (Method 1-2 of preparing acetone without using petroleum resources) were subjected to an aldol condensation reaction to synthesize diacetone alcohol. The diacetone alcohol was then easily dehydrated to be converted to mesityl oxide. The mesityl oxide was hydrogenated with a palladium catalyst to synthesize methyl isobutyl ketone. [0126] The following reaction was performed using the biomass-derived aniline obtained by the above method and nitrobenzene generated in that process. Meanwhile, nitrobenzene may be synthesized by oxidation of a part of the biomass-derived aniline according to a known method. An amount of 187 g of a 25% aqueous tetramethylammonium hydroxide solution (TMAOH) was concentrated by distillation at a temperature of 55°C under a pressure of 75 mbar to give a 35% solution. After addition of the biomass-derived aniline (269 mL) to the solution, the aniline/water azeotrope was evaporated at a temperature of 75°C under a pressure of 75 mbar until the molar ratio of water/base reached about 4:1. Subsequently, 60 g of the nitrobenzene was added and the resulting mixed solution was further stirred for four hours, while distillation of the water/aniline azeotrope was continued. To the crude mixed solution were added 2.2 g of a Pt/C catalyst (5% Pt) and 120 mL of water. Next, at a temperature of 80°C, the pressure was increased to the maximum of 15 bar with hydrogen, and then the reaction mixture was stirred until no further absorption of hydrogen was found. To the resulting mixture was added 100 mL of toluene, and the catalyst was removed by filtration, followed by separation of the mixture into an organic phase and a water phase with a separatory funnel. Then, purification of the organic phase by fractional distillation gave 4-aminodiphenylamine in a yield of 91%. An amount of 129.3 g of the 4-aminodiphenylamine, 120.2 g of methyl isobutyl ketone synthesized above, 0.77 g of a platinum catalyst (5% Pt on carbon sulfide powder (hydrous product), water content: 55.26% by mass, produced by N.E. Chemcat Corporation), and 0.65 g of activated carbon (Taiko activated carbon S-type, produced by Futamura Chemical Co., Ltd.) were introduced into a stirring autoclave and exposed to a hydrogen atmosphere. Then, the inside temperature of the autoclave was raised from room temperature to 150°C over about one hour. Subsequently, the hydrogen pressure was increased to 30 kgf/cm2 (2.94 MPa), and a reaction was allowed to proceed at the same temperature and the same pressure while feeding the consumed amount of hydrogen. After two hours from the start of increasing the hydrogen pressure, hydrogen was released from the autoclave to decrease the pressure to normal pressure, while the reaction solution was cooled to room temperature. The reaction solution was filtrated to remove the catalyst and the activated carbon. The resulting reaction product was subjected to separation by high performance liquid chromatography to give No.41 4-(1,3-dimethylbutylamino)diphenylamine (antioxidant 6PPD-1) in a yield of 99.4%.
References:
Sumitomo Rubber Industries, Ltd.;HATTORI Takayuki;WADA Takao;FUJIKURA Keitaro;YOKOYAMA Yuka;MATSUO Toshiro EP2543654, 2013, A1 Location in patent:Paragraph 0125; 0126
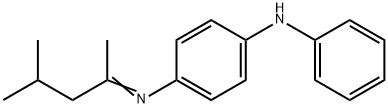
63302-40-9
0 suppliers
inquiry

793-24-8
271 suppliers
$5.00/1g

108-10-1
907 suppliers
$12.00/25ml

101-54-2
223 suppliers
$14.00/25g

793-24-8
271 suppliers
$5.00/1g

63302-40-9
0 suppliers
inquiry

108-10-1
907 suppliers
$12.00/25ml

101-54-2
223 suppliers
$14.00/25g

793-24-8
271 suppliers
$5.00/1g

62-53-3
689 suppliers
$10.00/1g
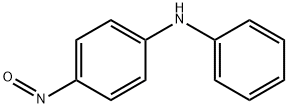
156-10-5
84 suppliers
$226.00/25g

108-10-1
907 suppliers
$12.00/25ml

793-24-8
271 suppliers
$5.00/1g