N-(2-Nitrophenyl)MethanesulfonaMide synthesis
- Product Name:N-(2-Nitrophenyl)MethanesulfonaMide
- CAS Number:85150-03-4
- Molecular formula:C7H8N2O4S
- Molecular Weight:216.21
Yield:85150-03-4 80%
Reaction Conditions:
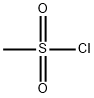
Stage #1: methanesulfonyl chloride;2-nitro-anilinewith pyridine at 20; for 18 h;
Stage #2: with sodium hydroxide in tetrahydrofuran;water at 20; for 2 h;
Stage #3: with hydrogenchloride in tetrahydrofuran;water; pH=7;
Steps:
1.f.i
Step (i) N-(2-nitrophenyl)methanesulfonamide Methanesulfonyl chloride (2.7 rnL, 48 mmol) was added to a solution of 2-nitroaniline (4.0 g, 29 mmol) in pyridine (10 ml), the mixture was stirred at room temperature for 18 h then poured over stirred ice. The precipitate was collected by filtration then dissolved in 2:3 THF / IM NaOH(aq) (100 mL) and stirred at room temperature for 2 h. The reaction mixture was acidified to pH 7 with 2M hydrochloric acid and extracted with ethyl acetate (3 x 30 mLl). The organics were washed with brine (30 mL) and water (30 mL), dried (MgSO4) and concentrated in vacuo to afford N- (2- nitrophenyljmethanesulfonamide as an orange solid (5.0 g, 80%). 1H NMR (dβ-DMSO) δ 9.82 (s, IH), 8.03 (d, IH), 7.75 (t, IH), 7.64 (d, IH), 7.41 (t, IH), 3.15 (t, 3H); LCMS method A, (ES+) 217, RT = 2.0 min.
References:
WO2009/112490,2009,A1 Location in patent:Page/Page column 53

108142-76-3
1 suppliers
inquiry

85150-03-4
20 suppliers
$172.00/50MG
3144-09-0
381 suppliers
$6.00/5g
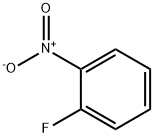
1493-27-2
511 suppliers
$5.00/10g

85150-03-4
20 suppliers
$172.00/50MG
1197-22-4
145 suppliers
$6.00/1g
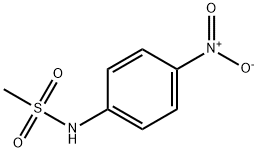
5825-62-7
32 suppliers
$28.60/50mg

85150-03-4
20 suppliers
$172.00/50MG
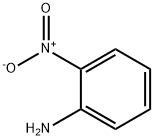
88-74-4
59 suppliers
$10.00/1g
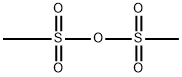
7143-01-3
280 suppliers
$19.00/5g

85150-03-4
20 suppliers
$172.00/50MG