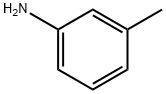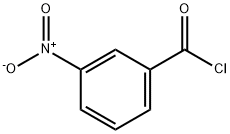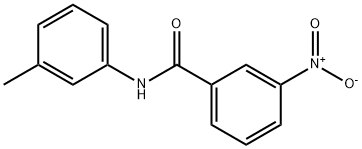
N-(3-methylphenyl)-3-nitrobenzamide synthesis
- Product Name:N-(3-methylphenyl)-3-nitrobenzamide
- CAS Number:69754-50-3
- Molecular formula:C14H12N2O3
- Molecular Weight:256.26
Yield:69754-50-3 96%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in dichloromethane at 20;Inert atmosphere;
Steps:
4.3.1. Preparation of 18
General procedure: To an ice-cooled solution of the appropriate acid chlorides (8,12.0 mmol) in DCM (10 mL), a solution of the corresponding aniline(16,12.0 mmol) followed by DIPEA (12.0 mmol) in DCM (15 mL) was added under an argon atmosphere. The reaction mixture was stirred at room temperature overnight and then concentrated under the reduced pressure. The residue was diluted in aqueous HCl solution(2-N, 50 mL) and extracted with EtOAc (2 x 75 mL). The combined organic phases were dried over anhydrous MgSO4 and concentrated under reduced pressure. The isolated solid was recrystallized in a suitable solvent mixture and dried in vacuum at 50 °C.
References:
Pillaiyar, Thanigaimalai;Funke, Mario;Al-Hroub, Haneen;Weyler, Stefanie;Ivanova, Sabrina;Schlegel, Jonathan;Abdelrahman, Aliaa;Müller, Christa E. [European Journal of Medicinal Chemistry,2020,vol. 186,art. no. 111789] Location in patent:supporting information
121-92-6
598 suppliers
$6.00/25g

69754-50-3
11 suppliers
inquiry