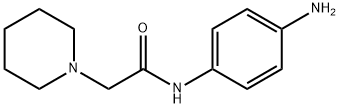
N-(4-aminophenyl)-2-piperidin-1-ylacetamide synthesis
- Product Name:N-(4-aminophenyl)-2-piperidin-1-ylacetamide
- CAS Number:100450-98-4
- Molecular formula:C13H19N3O
- Molecular Weight:233.31
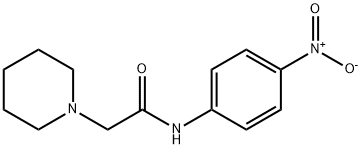
38367-22-5
3 suppliers
inquiry

100450-98-4
12 suppliers
$16.00/250mg
Yield:100450-98-4 98%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in ethyl acetate; under 1810.07 - 1965.21 Torr;
Steps:
N-(3-Aminophenyl)-3-(dimethylamino)propanamide (15a)
General procedure: To a solution of 14a (4.7 g, 20 mmol) in EA, 10% Pd/C (1.0 g) was suspended. The mixture was hydrogenated at 35 psi overnight. The reaction mixture was filtered through a pad of Celite. The filter cake was washed successively with EA and methanol. The combined filtrate and washings were evaporated in vacuo and dried to give 15a as a brown gum. Yield: 3.9 g (94%)
References:
Tala, Satishkumar D.;Ou, Tai-Hsin;Lin, Yi-Wen;Tala, Kiranben S.;Chao, Shu-Hsin;Wu, Ming-Hsi;Tsai, Tung-Hu;Kakadiya, Rajesh;Suman, Sharda;Chen, Ching-Huang;Lee, Te-Chang;Su, Tsann-Long [European Journal of Medicinal Chemistry,2014,vol. 76,p. 155 - 169] Location in patent:supporting information
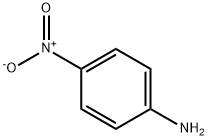
100-01-6
10 suppliers
$11.19/25G

100450-98-4
12 suppliers
$16.00/250mg
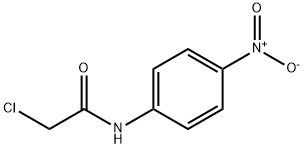
17329-87-2
58 suppliers
$24.00/1g

100450-98-4
12 suppliers
$16.00/250mg
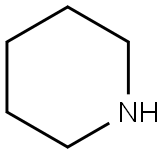
110-89-4
3 suppliers
$15.96/100ML

100450-98-4
12 suppliers
$16.00/250mg