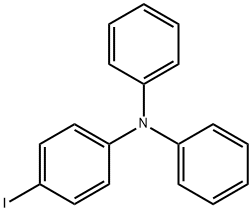
N-(4-IODOPHENYL)-N-PHENYLBENZENAMINE synthesis
- Product Name:N-(4-IODOPHENYL)-N-PHENYLBENZENAMINE
- CAS Number:38257-52-2
- Molecular formula:C18H14IN
- Molecular Weight:371.22
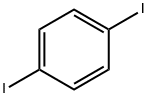
624-38-4
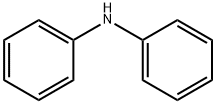
122-39-4

38257-52-2
GENERAL STEPS: 1,4-Diiodobenzene (396 mg, 1.2 mmol), diphenylamine (169 mg, 1 mmol), cuprous iodide (CuI, 19 mg, 0.1 mmol), phenanthroline (28.2 mg, 0.12 mmol) and potassium hydroxide (224 mg) were added to anhydrous toluene (20 mL) under nitrogen protection. The reaction mixture was heated to 120 °C and the reaction was stirred at this temperature for about 12 hours. Upon completion of the reaction, water (30 mL) was added to quench the reaction. The reaction mixture was extracted three times with dichloromethane (CH2Cl2, 30 mL), the organic phases were combined and dried overnight with anhydrous magnesium sulfate. The organic solvent was removed by evaporation under reduced pressure to give the crude product. The crude product was purified by silica gel column chromatography with petroleum ether as eluent to afford N-(4-iodophenyl)-N-phenylaniline (315.4 mg, 85%) as white powder. Melting point >300°C; 1H NMR (300 MHz, CDCl3): δ 7.58 (d, 2H), 7.28 (m, 4H), 7.08 (m, 6H), 6.83 (d, 2H); MS (ESI): m/z, calculated value for C18H14IN: 371.21, measured value: 372.02 [M + H]+.

62-53-3
691 suppliers
$10.00/1g

38257-52-2
114 suppliers
$6.00/100mg
Yield:-
Steps:
Multi-step reaction with 2 steps
1: copper(I) iodide; 1,7-phenanthroline; potassium hydroxide / toluene / Inert atmosphere; Reflux
2: potassium iodide; potassium iodate / acetic acid / Inert atmosphere; Reflux
References:
Safaei-Ghomi, Javad;Akbarzadeh, Zeinab [Ultrasonics Sonochemistry,2015,vol. 22,p. 365 - 370]

624-38-4
393 suppliers
$6.00/5g

122-39-4
357 suppliers
$14.00/5g

38257-52-2
114 suppliers
$6.00/100mg
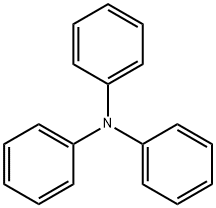
603-34-9
316 suppliers
$24.89/5g

38257-52-2
114 suppliers
$6.00/100mg

624-38-4
393 suppliers
$6.00/5g
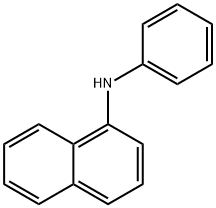
90-30-2
334 suppliers
$10.00/25g

38257-52-2
114 suppliers
$6.00/100mg
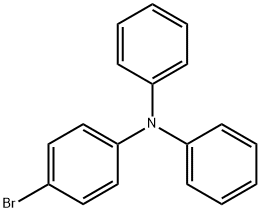
36809-26-4
302 suppliers
$29.00/5g

38257-52-2
114 suppliers
$6.00/100mg