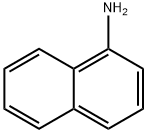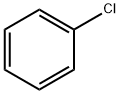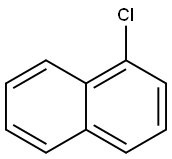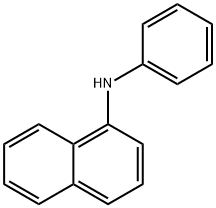
N-Phenyl-1-naphthylamine synthesis
- Product Name:N-Phenyl-1-naphthylamine
- CAS Number:90-30-2
- Molecular formula:C16H13N
- Molecular Weight:219.28
Yield:90-30-2 88%
Reaction Conditions:
in ethanol for 6 h;Reflux;
Steps:
2.3. Synthetic method for the preparation of N-phenylnaphthalen-1-amine2b
To a solution of naphthalen-1-amine (1.0 g, 6.98 mmol) in ethanol(3 mL), chlorobenzene (0.783 g, 6.98 mmol) was added, and the resultingmixture was refluxed for 6 h. The reaction progress was monitoredby TLC (dichloromethane/methanol, 9.5:0.5 vol). After completionof the reaction, solvent was evaporated and the mixture waspurified by column chromatography on silica using dichloromethaneand dichloromethane/methanol (99:1), as the eluent. N-phenylnaphthalen-1-amine 2b was obtained as pink solid (1.357 g, yield88%). Mp 59-61 °C. Rf=0.40 (dichloromethane/methanol,9.0:1.0 vol): 1H NMR (CDCl3, 400 MHz): δH 6.81 (dd, J=6.8 and1.6 Hz, 2 H, 2-H and 4-H Ph), 7.32-7.41 (m, 4 H, 3-H Ph, 5-H Ph, 2-H Phand 6-H Ph), 7.48-7.54 (m, 3 H, 4-H, 6-H, 3-H), 7.82-7.89 (m, 3 H, 7-H,5-H and 8-H) ppm. 13C NMR (CDCl3, 100.6 MHz): δC 109.61 (C-2 and1×Ar-C), 118.89 (2×Ar-C), 120.74 (2×Ar-C), 123.59 (C-8a),124.78 (1×Ar-C), 125.78 (1×Ar-C), 126.28 (3×ArC), 128.48(1×Ar-C), 134.33 (C-4a and C-1 Ph), 142.02 (C-1) ppm.
References:
Raju, B. Rama;Leitão, Maria Inês P.S.;Sousa, Maria João;Coutinho, Paulo J.G.;Gonçalves, M. Sameiro T. [Dyes and Pigments,2020,vol. 173,art. no. 107870]