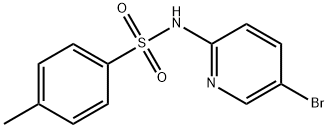
N-(5-bromopyridin-2-yl)-4-methylbenzenesulfonamide synthesis
- Product Name:N-(5-bromopyridin-2-yl)-4-methylbenzenesulfonamide
- CAS Number:207801-52-3
- Molecular formula:C12H11BrN2O2S
- Molecular Weight:327.2
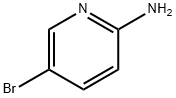
1072-97-5
697 suppliers
$9.00/1g

98-59-9
584 suppliers
$9.00/5g

207801-52-3
31 suppliers
$45.00/50mg
Yield:207801-52-3 100%
Reaction Conditions:
with pyridine at 0 - 90;
Steps:
4.1
Step 1) Formation of N-(5-bromopyridin-2-yl)-4-methylbenzenesulfonamideTo a solution of 2-amino-5-bromopyridine (40 g, 0.23 mol) in anhydrous pyridine (240 ml_), was slowly added tosyl chloride (53 g, 0.28 mol) at 00C. The reaction mixture was then heated at 900C for 16 hours. Pyridine was removed under reduced pressure and water (500 ml.) was added. The resulting mixture was stirred for 30 min at RT and filtered off to give the title compound as an off-white solid (77.0 g, quantitative). 1H-NMR (DMSO-d6, 400 MHz) δ 1 1.24 (brs, 1 H), 8.26 (d, 1 H), 7.89 (m, 1 H), 7.74 (m, 2H), 7.35 (m, 2H), 7.02 (d, 1 H), 2.36 (s, 3H).
References:
WO2010/100144,2010,A1 Location in patent:Page/Page column 103
30766-11-1
396 suppliers
$3.00/1g
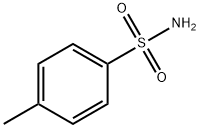
70-55-3
565 suppliers
$6.00/100g

207801-52-3
31 suppliers
$45.00/50mg