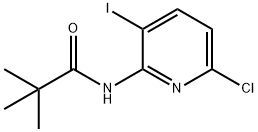
N-(6-chloro-3-iodopyridin-2-yl)pivalaMide synthesis
- Product Name:N-(6-chloro-3-iodopyridin-2-yl)pivalaMide
- CAS Number:800402-05-5
- Molecular formula:C10H12ClIN2O
- Molecular Weight:338.57
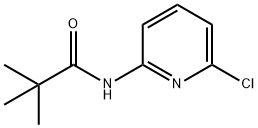
86847-84-9
92 suppliers
$22.00/250mg

800402-05-5
15 suppliers
inquiry
Yield:800402-05-5 78%
Reaction Conditions:
Stage #1: 2,2-Dimethyl-N-(6-chloro-2-pyridinyl)propanamide in tetrahydrofuran;pentane at -78; for 3.5 h;
Stage #2: with iodine in tetrahydrofuran;pentane at -78 - 20; for 2.16667 h;
Stage #3: with hydrogenchloride;water in tetrahydrofuran;pentane;
Steps:
B.1
B. 1 N-(6-chloro-3-iodopyridin-2-yl)pivalamide[0336] To a solution of N-(6-chloropyridin-2-yl)pivalamide (15.8 g, 74.2 mmol) in anhydrousTHF (150 mL) at -78 0C under a nitrogen atmosphere, is added 1.7 M -butyllithium in pentane (96 mL, 163 mmol, 2.2 eq.) dropwise (dropping funnel) over 0.5 h. The reaction mixture is then stirred at -78 0C for 3 h before iodine (22.6 g, 89 mmol, 1.2 eq.) in THF (60 mL) is slowly added in one portion. After 10 min., the cooling bath is removed and the reaction is allowed to warm to rt and stirred for 2 h. Hydrochloric acid (1 M, 75 mL) is then added to the reaction reaction mixture. The reaction mixture is concentrated in vacuo (rotary evaporator) to remove the THF, the resulting mixture is extracted with ethyl acetate (800 mL). The phases are separated and the organic layer is washed with aqueous 1 M Na2S2θ3 (100 mL), brine (300 mL x2), water (300 mL), dried over MgSO/t, and evaporated. The crude product is recrystalized from DCM/hexanes (1 :4) and the solid that forms collected by filtration to provide N-(6-chloro-3-iodopyridin-2-yl)pivalamide as a white crystalline solid (17.2 g). The filtrate is evaporated and the residue chromatographed on a silica gel column (hexanes/EtOAc, 9/1) to provide an additional product (2.5 g). Overall 19.7 g (78% yield) of N-(6-chloro-3-iodopyridin-2-yl)pivalamide is obtained. 1H NMR (300 MHz, DMSO-^6), δ 9.86 (s, IH), 8.30 (d, J = 8.4 Hz, IH), 7.20 (d, J = 8.4 Hz, IH), 1.23 (s, 9H). LCMS-ESI (m/z): calcd for C10H12ClIN2O 337.9; [M+H]+ found 339.0.
References:
WO2008/65198,2008,A1 Location in patent:Page/Page column 72
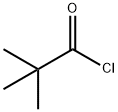
3282-30-2
380 suppliers
$22.00/100ml

800402-05-5
15 suppliers
inquiry
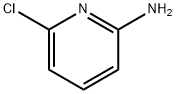
45644-21-1
374 suppliers
$10.00/5g

800402-05-5
15 suppliers
inquiry

45644-21-1
374 suppliers
$10.00/5g

3282-30-2
380 suppliers
$22.00/100ml

800402-05-5
15 suppliers
inquiry
141-86-6
515 suppliers
$6.00/5g

800402-05-5
15 suppliers
inquiry