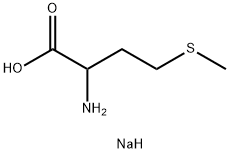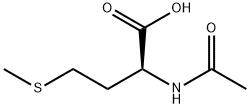
N-Acetyl-DL-methionine synthesis
- Product Name:N-Acetyl-DL-methionine
- CAS Number:1115-47-5
- Molecular formula:C7H13NO3S
- Molecular Weight:191.25
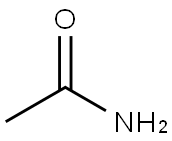
60-35-5
530 suppliers
$10.80/1g
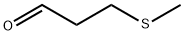
3268-49-3
290 suppliers
$25.39/5ml

1115-47-5
427 suppliers
$6.00/25g
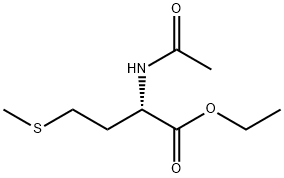
33280-93-2
28 suppliers
$244.00/500mg

24949-35-7
40 suppliers
$79.40/1g
Yield:1115-47-5 89.9% ,24949-35-7 4% ,33280-93-2 1%
Reaction Conditions:
Stage #1:acetamide;carbon monoxide with hydrogen;dicobalt octacarbonyl in ethyl acetate at 80; under 97509.8 Torr; for 0.0833333 h;
Stage #2:3-(methylsulfenyl)propanal in ethyl acetate at 80; under 97509.8 Torr; for 2.5 h;Product distribution / selectivity;
Steps:
2
Example 2 3.02 g of acetamide and 0.142 g of Co2 (CO)8, cobalt catalyst precursor, were dissolved in 20 ml of ethyl acetate in a 100 ml laboratory autoclave. The reactor was pressurised to 130 bar (130,000 hPa) with 1:1 Hz/CO synthesis gas and heated to 80° C whilst stirring. After 5 minutes a solution of 5.36 g MMP (97 %) in 25 ml of ethyl acetate was slowly added using an HPLC pump at a rate of 0.42 ml/min up to 50 % addition, 0.21 ml/min up to 75 % addition, 0.13 ml/min up to 91 % addition and 0.08 ml/min up to 100 % addition. Subsequently, 5 ml of ethyl acetate were added to the reaction in order to rinse the pump and addition line. The reaction was continued for a further 2.5 hours, after which the reactor vessel was cooled to room temperature and the pressure released. Analysis of the reaction mixture using HPLC gave: MMP conversion 96 % Yield (N-acetyl methionine) 89.9 % Selectivity (N-acetyl methionine) 93.6 % Side products included < 1 % N-acetyl methionine ethyl ester and approximately 4 % 1,3-bis(methylthio)propane.
References:
DEGUSSA AG WO2005/121079, 2005, A1 Location in patent:Page/Page column 12

60-35-5
530 suppliers
$10.80/1g
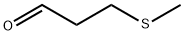
3268-49-3
290 suppliers
$25.39/5ml
201230-82-2
1 suppliers
inquiry

1115-47-5
427 suppliers
$6.00/25g

24949-35-7
40 suppliers
$79.40/1g

60-35-5
530 suppliers
$10.80/1g
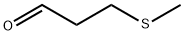
3268-49-3
290 suppliers
$25.39/5ml
201230-82-2
1 suppliers
inquiry

1115-47-5
427 suppliers
$6.00/25g

60-35-5
530 suppliers
$10.80/1g
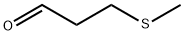
3268-49-3
290 suppliers
$25.39/5ml
201230-82-2
1 suppliers
inquiry

1115-47-5
427 suppliers
$6.00/25g

33280-93-2
28 suppliers
$244.00/500mg

24949-35-7
40 suppliers
$79.40/1g