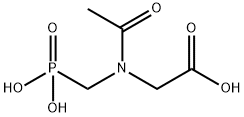
N-Acetyl Glyphosate synthesis
- Product Name:N-Acetyl Glyphosate
- CAS Number:129660-96-4
- Molecular formula:C5H10NO6P
- Molecular Weight:211.11

50-00-0
841 suppliers
$10.00/25g
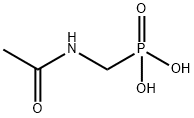
57637-97-5
20 suppliers
inquiry
201230-82-2
1 suppliers
inquiry

129660-96-4
21 suppliers
$40.00/2 mg
Yield: 94.5%
Reaction Conditions:
with hydrogenchloride;dicobalt octacarbonyl;water;hydrogen in tetrahydrofuran at 120; under 71257.1 Torr; for 2 h;Autoclave;Inert atmosphere;Solvent;pH-value;Reagent/catalyst;
Steps:
1; 2; 3
N-phosphorylmethylacetamide (93.7 g, 0.600 mol), paraformaldehyde (21.6 g, was added to the autoclave.0.72 mol), water (32.4 g, 1.80 mol), Co2 (CO) 8 (6.10 g, 0.0178 mol), co-catalyst hydrochloric acid, tetrahydrofuran(300mL). After replacing the nitrogen twice, it was charged with 95:5 CO/H2 and the pressure was 9.5 MPa. The mixture is heated to 120 ° C and stirred for 2 hours.The mixture was concentrated under reduced pressure to give N-acetyl glyphosamine, and the yield was calculated to be 94.5%. This step of organic solvent recovery is reused, no waste liquid is produced. By identifying the structure of the obtained N-acetylglyphosate, the results are as follows
References:
Shandong Runbo Biological Technology Co., Ltd.;Sun Guoqing;Hou Yongsheng;Xu Gaofei;Li Haihua;Li Hongli CN109942626, 2019, A Location in patent:Paragraph 0038; 0040-0041; 0045; 0047-0048; 0052; 0054-0055