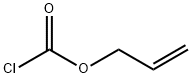
N-Alloc-L-Valine synthesis
- Product Name:N-Alloc-L-Valine
- CAS Number:115491-96-8
- Molecular formula:C9H15NO4
- Molecular Weight:201.22
Yield:115491-96-8 100%
Reaction Conditions:
with potassium carbonate in tetrahydrofuran;water at 20; for 18 h;
Steps:
i.a (S)-2-(allyloxycarbonylamino)-3-methylbutanoic acid
Allyl chloroformate (36.2 ml, 340.59 mmol, 1.2 eq) was added dropwise to a stirred solution of L-valine (I1 )(33.25 g, 283.82 mmol, 1 .0 eq) and potassium carbonate (59.27 g, 425.74 mmol, 1 .5 eq) in water (650 ml.) and THF (650 ml_). The reaction mixture was stirred at room temperature for 18 hours, then the solvent was concentrated under reduced pressure and the remaining solution extracted with diethyl ether (3 x 100 ml_). The aqueous portion was acidified to pH 2 with cone. HCI and extracted with DCM (3 x 100 ml_). The combined organics were washed with brine, dried over MgS04, filtered and concentrated under reduced pressure to afford the product as a colourless oil (57.1 g, assumed 100% yield). LC/MS (1 .966 min (ES+)), m/z: 202.1 [M+H]+. 1H NMR (400 MHz, DMSO-d6) δ 12.57 (br s, 1H), 7.43 (d, 1H, J=8.6 Hz), 5.96-5.86 (m, 1H), 5.30 (ddd, 1H, J= 17.2, 3.4, 1.7 Hz), 5.18 (ddd, 1H, J= 10.4, 2.9, 1.6 Hz), 4.48 (dt, 2H, J= 5.3, 1.5 Hz), 3.85 (dd, 1H, J= 8.6, 6.0 Hz), 2.03 (oct, 1H, J = 6.6 Hz), 0.89 (d, 3H, J= 6.4 Hz), 0.87 (d, 3H, J= 6.5 Hz),
References:
WO2013/53872,2013,A1 Location in patent:Page/Page column 90; 91
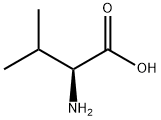
72-18-4
883 suppliers
$5.00/25g
135544-68-2
132 suppliers
$15.60/5G

115491-96-8
25 suppliers
inquiry

72-18-4
883 suppliers
$5.00/25g
115491-93-5
62 suppliers
$60.00/100mg

115491-96-8
25 suppliers
inquiry
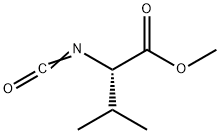
30293-86-8
42 suppliers
$70.56/1G

115491-96-8
25 suppliers
inquiry
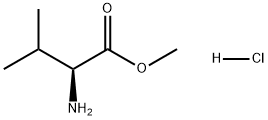
6306-52-1
557 suppliers
$5.00/5g

115491-96-8
25 suppliers
inquiry