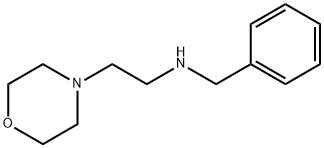
N-Benzyl-2-MorpholinoethanaMine synthesis
- Product Name:N-Benzyl-2-MorpholinoethanaMine
- CAS Number:2038-05-3
- Molecular formula:C13H20N2O
- Molecular Weight:220.31
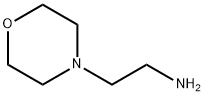
2038-03-1
206 suppliers
$27.00/25g
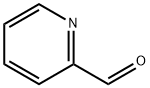
1121-60-4
499 suppliers
$10.60/10gm:

2038-05-3
18 suppliers
inquiry
Yield:2038-05-3 90%
Reaction Conditions:
Stage #1: 4-(2-AMINOETHYL)MORPHOLINE;pyridine-2-carbaldehyde in ethanol; for 12 h;Reflux;
Stage #2: with sodium tetrahydroborate in ethanol; for 2 h;Reflux;
Steps:
2.2.1. General synthesis of the amines (A 1 ) and (A 2 )
The general method for the synthesis of the amines is as pre- viously reported [ 21 , 23 ]. A solution of 2-morpholinoethanamine (1.30 g,10 mmol) in dry EtOH (30 mL) was slowly added dropwise to a warm solution of pyridine-2-carbaldehyde (2.14 g, 20.0 mmol) in dry EtOH (30 mL) for 2h. The mixture was refluxed under stirring for 12 h and then allowed to cool to room temperature. Solid sodium borohydride (3.78 g, 100 mmol) was then slowly added and the reaction mixture was heated at reflux for further 2 h. The solution was filtered and the filtrate volume decreases to 20 mL by rotary evaporation. Excess water was added and the product was then extracted with chloroform (3 ×25 mL); the combined chloroform solutions were separated and dried over magnesium sulfate. The chloroform was removed by ro- tary evaporation to leave a yellow oil ((90%). The resulting prod- uct (10 mmol) was dissolved in acetonitrile (20 mL), and K 2 CO 3 (2.76 g, 20 mmol) was added. The mixture was refluxed and then a solution of N-(3-bromopropyl)phthalimide (3.22 g, 12.0 mmol) or N-(4-bromobuthyl)phthalimide (3.38 g, 12.0 mmol) in acetonitrile (40 mL) was added. The mixture was refluxed for 24 h and then fil- tered while still hot. The filtrate was reduced to dryness by rotary evaporation and the resulting product was heated under reflux for 20 h in aqueous HCl (25%, 100 mL). The solution was then evapo- rated to a low volume (ca. 25 mL) under vacuum and then cooled in a refrigerator for several hours. The solid product was filtered off, and the filtrate was evaporated to dryness under vacuum. Wa- ter (50 mL) was added to the mixture and the pH was adjusted to 12 with sodium hydroxide. The product was then extracted with chloroform (3 ×25 mL), and then the combined chloroform solutions were separated and dried over magnesium sulfate. The chlo- roform was removed by rotary evaporation to leave the product as a brown oil
References:
Rezaeivala, Majid;Sayin, Koray;Zebarjadian, Mohammad Hasan [Journal of Molecular Structure,2021,vol. 1242,art. no. 130703]

2038-03-1
206 suppliers
$27.00/25g

100-46-9
460 suppliers
$5.00/5G

2038-05-3
18 suppliers
inquiry
103-49-1
432 suppliers
$5.00/25g

17785-41-0
5 suppliers
inquiry

2038-05-3
18 suppliers
inquiry

2038-03-1
206 suppliers
$27.00/25g

2038-05-3
18 suppliers
inquiry

100-52-7
946 suppliers
$21.30/2g

2038-05-3
18 suppliers
inquiry