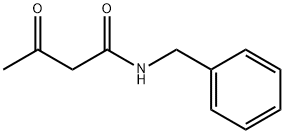
N-Benzylacetoacetamide synthesis
- Product Name:N-Benzylacetoacetamide
- CAS Number:882-36-0
- Molecular formula:C11H13NO2
- Molecular Weight:191.23
Yield: 100%
Reaction Conditions:
in dichloromethane at 0 - 20;
Steps:
2.1 Step 1) jV-benzyl-3-oxobutanamide
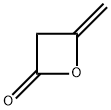
[325] To a solution of 4-methyleneoxetan-2-one (60.00 g, 713.69 mmol) in DCM (600 mL) was added phenylmethanamine (91.76 g, 856.37 mmol) dropwise at 0 °C. The reaction was moved to room temperature and stirred overnight, then concentrated in vacuo. The residue was diluted with a mixture of PE and EtOAc (10/1 (v/v), 200 mL) and stirred at room temperature for 1 h, then the mixture was filtered. The filter cake was washed with a mixture of PE and EtOAc (10/1 (v/v), 100 mL) to give the title compound as a yellow solid (136.48 g, 100 %). MS (ESI, pos. ion) m z: 192.3 [M+H]+; NMR (400 MHz, CDCI3): δ 7.43 (s, 1H), 7.34-7.21 (m, 5H), 4.41 (d, J = 5.8 Hz, 2H), 3.38 (s, 2H), 2.21 (s, 3H).
References:
CALITOR SCIENCES, LLC;SUNSHINE LAKE PHARMA CO., LTD.;XI, Ning;LI, Minxiong;HU, Haiyang;WANG, Tingjin WO2015/94803, 2015, A1 Location in patent:Paragraph 325