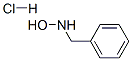
N-Benzylhydroxylamine hydrochloride synthesis
- Product Name:N-Benzylhydroxylamine hydrochloride
- CAS Number:29601-98-7
- Molecular formula:C7H10ClNO
- Molecular Weight:159.61
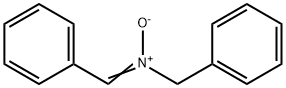
3376-26-9
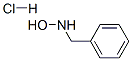
29601-98-7
The general procedure for the synthesis of N-benzylhydroxylamine hydrochloride from the compound (CAS:3376-26-9) was as follows: in a two-necked round-bottomed flask equipped with a stirrer, a mixture of dibenzylamine (1) (32.8 g, 0.17 mol), Na2WO4-2H2O (0.98 g, 3.33 mmol) and methanol (0.17 L) was cooled to -15 °C. A 30% aqueous solution of H2O2 (57 mL, 0.5 mol) was slowly added dropwise over about 1 h via a pressure equalizing dropping funnel. After the dropwise addition, the reaction mixture was stirred continuously at room temperature for 16 hours. Subsequently, the mixture was transferred to a 3 L beaker and crushed ice (1 kg) was added under vigorous stirring. The white precipitate formed was collected by filtration and washed several times with ice water (1 L) to completely remove the peroxide. The wet crude nitrone was dissolved in 20% aqueous HCl solution (0.33 L) and concentrated under reduced pressure at a water bath temperature of 70 °C. To remove trace water, the mixture was washed with toluene (3 x 33 mL) and subsequently concentrated under vacuum to dryness overnight. Finally, recrystallization was carried out using minimal amounts of hot methanol (30 mL) and ether (115 mL) to give a white crystalline pure product (19.3 g, 71% yield). The 1H and 13C NMR spectra of the product were in agreement with those reported in the literature; ESI-HRMS analysis resulted in a calculated value of 124.07 and a measured value of 124.0750 for C7H9NO(M + H)+.
57079-14-8
29 suppliers
inquiry
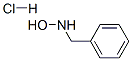
29601-98-7
292 suppliers
$5.00/5g
Yield:29601-98-7 87.3%
Reaction Conditions:
with hydroxylamine hydrochloride in ethanol;tert-butyl methyl ether at 35; for 3 h;
Steps:
1.2 Step 2: Synthesis of N-benzylhydroxylamine hydrochloride
Take a 5L three-necked bottle, install a thermometer, and stir mechanically.Add C-phenyl-N-benzylnitrone (535.7g, content 98.0%, 2.46mol),Add ethanol (197.3g, 4.28mol),Add methyl tert-butyl ether (1849.9g, 20.98mol),Warm up to 35°C, stir to dissolve,Add hydroxylamine hydrochloride (182.9g, 2.63mol),The reaction was kept at 35°C for 3 hours, and the C-phenyl-N benzyl nitrone was controlled to be ≤2.0%.Cool down to 0, keep for 2 hours to crystallize,Filter, wash the filter cake with methyl tert-butyl ether,The crude N-benzylhydroxylamine hydrochloride was obtained.Take another 2L three-necked bottle, install a thermometer, and stir mechanically.Add crude N-benzyl hydroxylamine hydrochloride,Add dichloromethane (2195.6g, 25.85mol),25 heat preservation and beating for 2 hours,Filter, wash the filter cake with dichloromethane, dry,Obtain N-benzylhydroxylamine hydrochloride (343.2g, 2.15mol),The yield was 87.3%, and the purity was 99.53%.
References:
CN113292446,2021,A Location in patent:Paragraph 0076; 0078; 0083-0086
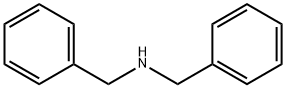
103-49-1
474 suppliers
$5.00/25g
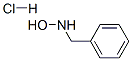
29601-98-7
292 suppliers
$5.00/5g

57079-14-8
29 suppliers
inquiry
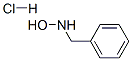
29601-98-7
292 suppliers
$5.00/5g

932-90-1
151 suppliers
$17.67/1gm:

932-90-1
151 suppliers
$17.67/1gm:
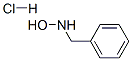
29601-98-7
292 suppliers
$5.00/5g
622-30-0
98 suppliers
$28.00/1g
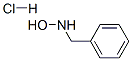
29601-98-7
292 suppliers
$5.00/5g