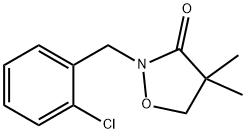
Clomazone synthesis
- Product Name:Clomazone
- CAS Number:81777-89-1
- Molecular formula:C12H14ClNO2
- Molecular Weight:239.7
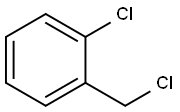
611-19-8
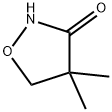
81778-07-6

81777-89-1
1. 1000 kg of water was added to a 4000 L reactor followed by 460 kg of 4,4-dimethylisoxazolidin-3-one. The mixture was stirred for 1 hour at room temperature. 2. 383kg of sodium carbonate (Na2CO3) was added to the reaction mixture in small portions. The temperature of the mixture was raised to 85°C and the mixture was stirred continuously at this temperature for 2 hours. 3. 672 kg of o-chlorobenzyl chloride (2-chlorobenzyl chloride) was added slowly and dropwise over a period of 5 hours at 85°C. The mixture was kept at the same temperature for 2 hours. After the dropwise addition was completed, the temperature was maintained at 85°C until the reaction was complete. 4. The reaction mixture was cooled to room temperature, 800 kg of dichloromethane was added and stirred at room temperature for 15 hours. 5. The aqueous phase was separated and the aqueous phase was extracted three times with dichloromethane. 6. Recover the dichloromethane by distillation followed by addition of 2000 kg of hexane to the reactor. The mixture was refluxed for 1 hour, then cooled to 10-15 °C and continued stirring for 1 hour. 7. The solid product was separated by filtration, washed several times with hexane and finally dried under high vacuum to give pure 2-(2-chlorobenzyl)-4,4-dimethyloxazolidin-3-one (815 kg, purity: 96%). Note: Similar results were obtained using sodium hydroxide instead of sodium carbonate as base.

611-19-8
472 suppliers
$7.00/25g

81778-07-6
66 suppliers
$61.00/100mg

81777-89-1
225 suppliers
$39.00/50mg
Yield:81777-89-1 973 kg
Reaction Conditions:
with potassium carbonate; pH=7 at 85; for 3 h;Large scale;Reagent/catalyst;
Steps:
1.4; 2.4; 3.4; 4.4 Step four, configuration conversion:
the crude isoxalinone in step 3, first dehydrated under reduced pressure, and then the dried oily mixture is cooled to 45 ° C, then passed through a dry hydrogen chloride gas, and maintained at this temperature for 3 h, Then, 10.5 kg of o-chlorobenzyl chloride, 9.57 kg of 30% by mass of liquid alkali, 2.9 kg of potassium carbonate solid were added to the reaction solution, and the pH was adjusted to 7.00, and then the temperature was raised to 85 ° C for 3 hours, and the amount of o-chlorobenzyl chloride was qualified. After standing, the layers were separated, the lower organic phase was separated, dehydrated under reduced pressure, and the oxaloxone product was distilled off under vacuum, and further purified by petroleum ether to obtain 973 kg of clomazone, content 98.1%. The yield (calculated as hydroxylamine hydrochloride) was 92.1%.
References:
Jiangsu Heben Biochemical Co., Ltd.;Zhejiang Heben Technology Co., Ltd.;Chen Hua;Deng Guiyuan;Jia Lihua;Zeng Ting;Pan Guangfei CN110172042, 2019, A Location in patent:Paragraph 0025; 0028; 0031; 0034; 0037; 0040
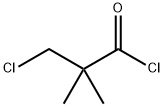
4300-97-4
219 suppliers
$53.00/5 g

611-19-8
472 suppliers
$7.00/25g

81777-89-1
225 suppliers
$39.00/50mg

611-19-8
472 suppliers
$7.00/25g
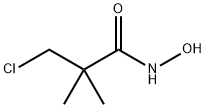
81778-06-5
35 suppliers
inquiry

81777-89-1
225 suppliers
$39.00/50mg
![Propanamide, 3-chloro-N-[(2-chlorophenyl)methyl]-N-hydroxy-2,2-dimethyl-](/CAS/20210111/GIF/81777-71-1.gif)
81777-71-1
0 suppliers
inquiry

81777-89-1
225 suppliers
$39.00/50mg
![Propanamide, 3-chloro-N-[(2-chlorophenyl)methyl]-N-hydroxy-2,2-dimethyl-](/CAS/20210111/GIF/81777-71-1.gif)
81777-71-1
0 suppliers
inquiry

4300-97-4
219 suppliers
$53.00/5 g

81777-89-1
225 suppliers
$39.00/50mg