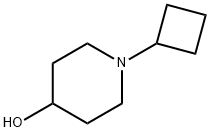
N-Cyclobutyl-4-hydroxypiperidine synthesis
- Product Name:N-Cyclobutyl-4-hydroxypiperidine
- CAS Number:869224-62-4
- Molecular formula:C9H17NO
- Molecular Weight:155.24
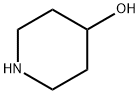
5382-16-1
0 suppliers
$9.00/5G
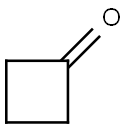
1191-95-3
329 suppliers
$10.00/1g

869224-62-4
33 suppliers
$130.00/250MG
Yield:869224-62-4 98.49%
Reaction Conditions:
Stage #1: 4-HYDROXYPIPERIDINE;cyclobutanone in 1,2-dichloro-ethane at 20 - 25; for 1.75 h;Large scale;
Stage #2: with sodium tris(acetoxy)borohydride in 1,2-dichloro-ethane at 15 - 30; for 15.4333 h;Large scale;
Steps:
1.i Step (i): Preparation of 1-cyclobutylpiperidin-4-oI
Step (i): Preparation of 1-cyclobutylpiperidin-4-oI V Ethylene dichloride (235 L) was charged into the reactor at 20-25 °Cfollowed by 4-hydroxy piperidine (9.5 Kg, 93.92 M). The mass was stirred for -15 minutes to obtain a clearsolution. Then cyclobutanone (7.9 Kg, 112.71 M) wascharged into the reactor at 20-25 °C and stirred the mass for9O minutes at the same temperature. The mass was cooled to 15-20 °C and started lot wise addition of sodium triacetoxy borohydride (39.9 Kg, 188.26 M) maintaining the mass temperature below 25 °C in - 110 minutes. After completionof addition, the masswas stirred for 30 minutes at - 20 °C. The mass temperature was raised to 25-30°C and maintained at the same temperature for 13.1 hours, while monitoring the progress of the reaction by Thin Layer Chromatography (TLC). After completion of the reaction, water (112 L) was charged into the reactor at 25-30 °C. The mass was then cooled to 15-20 °C and pH of the reaction mass was adjusted to 13.0-13.5 with a solution of aqueous sodium hydroxide (24.6 Kg of sodium hydroxide dissolved in 106 L of demineralised water (DM water) maintaining the mass temperature below 20 °C in about 1 hour 20 minutes. In the meanwhile, nutsche filter with hyflow bed (using 4.75 Kg hyflow and 47.5 L DM water) was made ready for filtration of dirt and sodium acetate salt, for the purpose of clean layer separations during extraction of the product. The reaction mass was filteredthrough nutsche and the nutsche was washed with 23.75 L of ethylene dichloride. The filtrate containing the product was collected into clean and dedicated containers. The combined filtrate and washings were transferred to a reactor, stirred 15 minutes and settled for 15 minutes at 25-30 °C. The bottom organic layer (containing the product) was collected in dedicated containers and the masswas dried over anhydrous sodium sulfate (9.5 Kg). The supernatant, clean, dry organic layer was taken in a reactor and solvent was removed by distillation under vacuum maintaining mass temperature below 50 °C. The residual crude mass was cooled to 25-3 0 °C.2H extraction of the aqueous layer: The aqueous layer separated as above wastaken in a reactor and charged dichloromethane (DCM) (56 L) at 25-3 0 °C. The mass was stirred 15 minutes and settled for 15 minutes. The bottom organic layer (containing product) was separated into dedicated containers. The aqueous layer was collected and taken for 3rd extraction.3rd extraction of the aqueous layer: The aqueous layer: separated as above wastakenin a reactor and charged DCM (56 L)at 25-30 °C. The mass was stirred 15 minutes and settled for 15 minutes. The bottom organic layer (containing product) was separated into dedicated containers. The aqueous layer was collected andtaken for 4th extraction.4th extraction of the aqueous layer: The aqueous layer: separated as above wastaken in a reactor and charged DCM (56 L) at 25-30 °C. The mass was stirred 15 minutes and settled for 15 minutes. The bottom organic layer (containing product) was separated into dedicated containers. The aqueous layer was collected andtaken for 5th extraction.5th extraction of the aqueous layer: The aqueous layer-separated as above wastaken in a reactor and charged dichioromethane (56 L) at 25-30 °C. The mass was stirred 15 minutes and settled for 15 minutes: The bottom organic layer (containing product) was separated into dedicated containers. The aqueous layer was collected in dedicated containers and kept aside.The organic layer obtained from second extraction to fifth extraction was combined and dried over anhydrous sodium sulfate (13.5 Kg). The supernatant,clean, dry organic layer was taken in the reactor, containing the crude product obtained from first extraction, and solvent was removed by distillation under reduced pressure (>500 mm Hg) maintaining mass temperature below 50 °C. The residual mass was cooled to 25-30 °C and collected the technical product (14.36 Kg).Yield: 98.49 %;‘H-NMR ( ppm, CDC13): 1.55 - 1.69 (5H, m), 1.83 - 2.02 (8H, m), 2.65 - 2.69 (3H, m), 3.66 - 3.70 (1H, m);Mass (mlz): 156.2 (M+H).
References:
WO2016/27275,2016,A1 Location in patent:Page/Page column 5; 6; 7

5382-16-1
0 suppliers
$9.00/5G
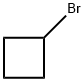
4399-47-7
232 suppliers
$14.14/1gm:

869224-62-4
33 suppliers
$130.00/250MG