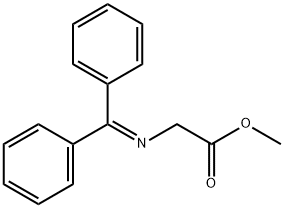
N-(DIPHENYLMETHYLENE)GLYCINE METHYL ESTER synthesis
- Product Name:N-(DIPHENYLMETHYLENE)GLYCINE METHYL ESTER
- CAS Number:81167-39-7
- Molecular formula:C16H15NO2
- Molecular Weight:253.3
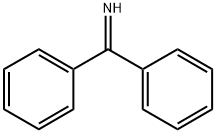
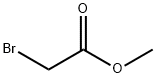
1013-88-3
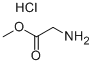
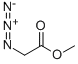
5680-79-5

81167-39-7
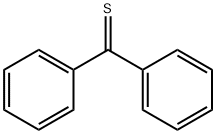
Benzophenone imine (25.00 g, 137 mmol) was added in a single addition to a stirring solution of glycine methyl ester hydrochloride (17.49 g, 139 mmol) in anhydrous dichloromethane (150 mL) under nitrogen atmosphere and at room temperature. The reaction mixture was stirred continuously for 24 h, during which ammonium chloride precipitation was observed to be generated. Upon completion of the reaction, water (20 mL) was added for layering. The organic layer was washed sequentially with saturated sodium carbonate solution (2 x 20 mL) and brine (20 mL). The organic layer was dried over anhydrous sodium sulfate, filtered and concentrated using a rotary evaporator to give about 35 g of a thick light brown slurry (99% purity) in near 100% yield. The product did not require further purification and could be used directly in the subsequent reaction.
Yield: 70%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in acetonitrile at 90; for 12 h;
Steps:
501
FIG. 5 shows an example of a process for synthesizing 1-(4-ethyl-8-methyl-7,8,9a,10-tetrahydropyrido[1,2-α]indole-6,9-dione). Step 501. A solution of methyl bromoacetate (5.5 g, 35.9 mmol) in acetonitrile (40 mL) was treated with benzophenonimine (6.5 g, 35.8 mmol) and diisopropylethylamine (6.2 mL, 4.6 g, 35.6 mmol). The resulted mixture was heated at reflux (90° C.) for 12 hours. After reaction completion, the mixture was cooled to room temperature and concentrated in vacuo. The formed residue was partitioned between water (40 mL) and diethyl ether (60 mL), and the organic portion was separated, dried over MgSO4, filtered, and concentrated in vacuo. The product was purified by flash column chromatography and eluted with ethyl acetate/hexane to obtain white crystals (70%).
References:
Texas Tech University System;German, Nadezhda;Hossain, Modammad Anwar US2020/131183, 2020, A1 Location in patent:Paragraph 0046

1013-88-3
393 suppliers
$10.00/5g

5680-79-5
546 suppliers
$3.66/25g

81167-39-7
148 suppliers
$9.00/5g
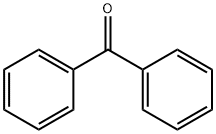
119-61-9
821 suppliers
$5.00/10g

5680-79-5
546 suppliers
$3.66/25g

81167-39-7
148 suppliers
$9.00/5g