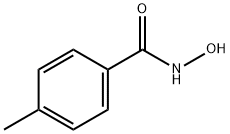
N-Hydroxy-4-methylbenzamide synthesis
- Product Name:N-Hydroxy-4-methylbenzamide
- CAS Number:2318-82-3
- Molecular formula:C8H9NO2
- Molecular Weight:151.16
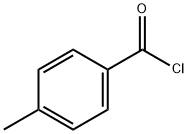
874-60-2
414 suppliers
$10.00/5g

2318-82-3
11 suppliers
inquiry
Yield:2318-82-3 100%
Reaction Conditions:
with hydroxylamine hydrochloride;sodium hydrogencarbonate in water;ethyl acetate at 20; for 0.0833333 h;
Steps:
synthesis of methylbenzohydroxamic acid, CH3-BHA
The synthesis of CH3-BHA is represented in Scheme 1.hydroxylamine hydrochloride (12 mmol, 0.9555 g) was added to a mixture solution of ethyl acetate and water containing sodium hydrogen carbonate (NaHCO3). The mixture was stirred at room temperature. 4-methylbenzoyl chloridewas diluted with small amounts of ethyl acetate was added dropwise to the mixture. The mixture was stirred for 5 min at room temperature. The solvent was removed in vacuo to afford the pure product. Peach solid; yield, 100%; m.p.152-154°C. Anal. Calcd. for C8H9NO2(151.20 gmol-1):C, 63.56; H, 6.00; N, 9.27; Found: C, 62.71; H, 5.70; N,9.00. IR (KBr, cm-1): 3294 (N-H), 2759 (O-H), 1651(C = O), 903 (N-O). 1H NMR (600 MHz, ppm, DMSO):δ = 2.41 (3H, s, CH3),7.23-7.62 (2H, d, Ar-H), 7.62 (2H,d, Ar-H). 13C NMR (600 MHz, ppm, DMSO): δ = 164.03,140.61, 130.26, 128.76, 126.73, 20.89. MS (m/z): Calcd forCH3-BHA: 152.20 [M + H]+; Found: 152.07 [M+H]+.
References:
Abdullah, Faiezah;Anouar, El Hassane;Bahron, Hadariah;Hassan, Latifah Robbaniyyah;Mohd Tajuddin, Amalina [Journal of Biological Inorganic Chemistry,2020]
99-94-5
610 suppliers
$9.00/1g

2318-82-3
11 suppliers
inquiry
99-75-2
286 suppliers
$6.00/10g

2318-82-3
11 suppliers
inquiry
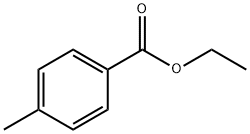
94-08-6
242 suppliers
$6.00/5g

2318-82-3
11 suppliers
inquiry
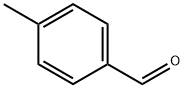
104-87-0
516 suppliers
$6.00/25g

2318-82-3
11 suppliers
inquiry