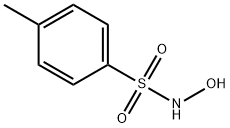
N-Hydroxy-4-methylbenzenesulfonamide synthesis
- Product Name:N-Hydroxy-4-methylbenzenesulfonamide
- CAS Number:1593-60-8
- Molecular formula:C7H9NO3S
- Molecular Weight:187.22

98-59-9

1593-60-8
General procedure for the synthesis of N-hydroxy-4-methylbenzenesulfonamide from p-toluenesulfonyl chloride: Hydroxylamine hydrochloride (7.2 g, 86 mmol) was dissolved in a solvent mixture of methanol (30 mL) and water (20 mL), followed by the addition of magnesium oxide (5.1 g, 129 mmol), and the reaction was stirred for 10 minutes. Next, a solution of tetrahydrofuran (300 mL) of p-toluenesulfonyl chloride (8 g, 43 mmol) was added to the above mixture and the reaction was vigorously stirred at room temperature. The progress of the reaction was monitored by thin layer chromatography (TLC) until the raw material was completely consumed. Upon completion of the reaction, the reaction mixture was filtered through diatomaceous earth and the filtrate was dried with anhydrous magnesium sulfate and subsequently concentrated under reduced pressure to afford N-hydroxy-4-methylbenzenesulfonamide (7 g, white solid) in 87.5% yield.

98-59-9
625 suppliers
$9.00/5g

1593-60-8
44 suppliers
$45.00/250mg
Yield: 87.5%
Reaction Conditions:
with hydroxylamine hydrochloride;magnesium oxide in tetrahydrofuran;methanol;water at 20;
Steps:
3.1 first step
Hydroxylamine hydrochloride (7.2 g, 86 mmol) was dissolved in methanol (30 ml) and water (20 ml), magnesium oxide (5.1 g, 129 mmol) was added, stirred for 10 minutes, then p-toluenesulfonyl chloride (8 g, 43 mmol) in tetrahydrofuran (300 ml), stirred vigorously at room temperature, and reacted to TLC to monitor the starting material for complete reaction. The extract was filtered through Celite, dried over anhydrous magnesium sulfate and concentrated under reduced pressure to give N-hydroxy-4-methylbenzenesulfonamide (7 g, white solid) in 87.5% yield.
References:
Shanghai Acebright Pharmaceuticals Group Co., Ltd.;An, Xiaoxia;Bie, Pingyan;Liu, Jun;Zhuang, Geshi CN105418632, 2016, A Location in patent:Paragraph 0175; 0176; 0177
70-55-3
589 suppliers
$6.00/100g

1593-60-8
44 suppliers
$45.00/250mg
66021-66-7
0 suppliers
inquiry

1593-60-8
44 suppliers
$45.00/250mg