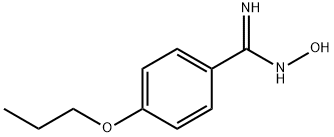
N'-Hydroxy-4-propoxybenzenecarboximidamide synthesis
- Product Name:N'-Hydroxy-4-propoxybenzenecarboximidamide
- CAS Number:145259-49-0
- Molecular formula:C10H14N2O2
- Molecular Weight:194.23
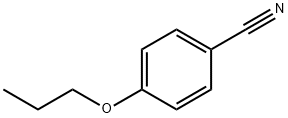
60758-84-1
61 suppliers
$15.00/500mg

145259-49-0
19 suppliers
$120.00/1g
Yield:145259-49-0 14.2 g
Reaction Conditions:
with dmap;hydroxylamine hydrochloride;copper hydroxide;potassium hydroxide in methanol at 0 - 50; under 60006 Torr; for 5 h;Inert atmosphere;Sealed tube;Supercritical conditions;
Steps:
1 Example 1
Under nitrogen protection, 14 g of hydroxylamine hydrochloride and 50 mL of anhydrous methanol were added to the four-necked flask. The reaction temperature was about 0 ° C, and 100 mL of a methanol solution containing 17 g of potassium hydroxide was slowly added dropwise.After the dropwise addition, rapidly stir and filter the reaction solution. The reaction solution was added to a supercritical reactor, and then 16 g of 4-propoxybenzonitrile and 14.5 g of 4-dimethylaminopyridine and 2 g of copper hydroxide were added, and the heating device in the reaction vessel was examined. Whether the induction device is normal, close the valve and check the air tightness; Turn on the cooling circulating water, turn on the controller main power, display power, and agitator power in turn, set the stirring speed, and set the reaction temperature. When the temperature in the reactor reaches 50 °C, open the carbon dioxide cylinder, open the inlet valve, open the carbon dioxide pump, pass carbon dioxide, and record the mass of the carbon dioxide passed through the mass flow meter: observe the reaction tank pressure reaches 8Mpa, then the reaction The material is completely dissolved, the pressure condition is maintained, the timing is started, and the temperature and pressure changes in the reaction vessel are observed during the reaction, and the temperature in the reaction vessel is adjusted with condensed water to ensure a constant reaction temperature; After reacting for 5 hours, the carbon dioxide in the reaction vessel was slowly released, and the heating was stopped. After the pressure was released completely, the reactor was opened, the reaction solution was filtered, the reaction solution was poured into 200 mL of water, and the reaction solution was extracted with ethyl acetate several times to combine organic phase, concentrated to give 14.2 g of N-hydroxy-4-propoxybenzylhydrazine;
References:
CN109776443,2019,A Location in patent:Paragraph 0023-0025