
N-Methyl-2-(methylamino)acetamide HCl synthesis
- Product Name:N-Methyl-2-(methylamino)acetamide HCl
- CAS Number:51739-63-0
- Molecular formula:C4H11ClN2O
- Molecular Weight:138.5959
Yield:51739-63-0 66%
Reaction Conditions:
in ethanol;water at 5 - 100;
Steps:
2.2. Preparation of N′-methylsarcosinamide hydrochloride,CH3NH2+CH2CONHCH3 Cl- (SarMeam · HCl)
Caution This preparation should be performed under a fume hood.Ethyl chloroacetate (12.2 g; 10.5 mL; 0.1 mol) was added to an excessof concentrated aqueous methylamine solution (80 mL; 40% w/w)contained in a 500 mL Erlenmeyer flask [Note 1]. A strongly exothermicreaction took place after about half a minute and the reaction mixturestarted to boil. The clear colourless solution was then left to stand forhalf an hour and then evaporated at 100 °C until the product started tocrystalize (the final volume was about 30 mL). After the mixturespontaneously cooled to ca 70 °C, ethanol (200 mL) was slowly addedwhile stirring. The mixture was left to stand overnight in a refrigerator(5 °C), the separated N′-methylsarcosinamide hydrochloride was filteredoff by suction, washed with cold ethanol (100 mL) and air-dried[Note 2]. The product was pure enough to be used without any furtherpurification.Yield: 9.0 g (66%), m.p. 234 °C. IR data (ATR, cm-1): 3237(w),3107(w), 2997(w), 2935(w), 2780(w), 2711(w), 2436(w), 1671(vs),1567(m), 1461(w), 1418(m), 1397(w), 1368(m), 1272(m), 1156(w),1069(w), 1042(w), 947(m), 903(w), 864(m), 725(m), 583(w). 1H NMR(600 MHz, DMSO-d6), δ/ppm: 2.53(s, 3H; CH3-NH2+), 2.64(d, 3H,J=4.62 Hz; CONH-CH3), 3.66(s, 2H; CH2-CO), 8.69(s, br, 1H; CONH),9.17(s, br, 2H; NH2+). 13C NMR (150 MHz, DMSO-d6), δ/ppm:25.32(CH3), 32.61(CH3), 48.85(CH2), 165.22(CONH).Note 1. Since the reaction is very vigorous, it is not advisable to scaleup the quantities of the reactants. A large volume of the flask is necessaryto prevent the reaction mixture to boil out.Note 2. An additional amount (1-2 g) of the product can be obtainedand isolated in a similar manner after evaporating the filtrate to10-20 mL.
References:
Smre?ki, Neven;Ron?evi?, Tomislav;Jovi?, Ozren;Kukovec, Boris-Marko;Maravi?, Ana;Gajski, Goran;?ike?-?uli?, Vedrana [Inorganica Chimica Acta,2019,vol. 488,p. 312 - 320]
![Carbamic acid, methyl[2-(methylamino)-2-oxoethyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/20200611/GIF/144332-55-8.gif)
144332-55-8
5 suppliers
inquiry

51739-63-0
12 suppliers
inquiry
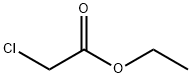
105-39-5
427 suppliers
$10.00/5g

74-89-5
0 suppliers
$13.44/25ML
107-97-1
603 suppliers
$5.00/100mg

51739-63-0
12 suppliers
inquiry