Sarcosine synthesis
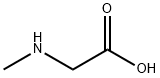
- Product Name:Sarcosine
- CAS Number:107-97-1
- Molecular formula:C3H7NO2
- Molecular Weight:89.09
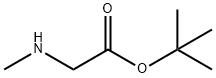
37429-48-4
30 suppliers
$99.00/250mg

107-97-1
600 suppliers
$5.00/100mg
Yield:107-97-1 100 %
Reaction Conditions:
with Pd/C;hydrogen under 760.051 Torr;
Steps:
General Procedure D
General procedure: Iridium complex 11 (3.2 mg, 0.005 mmol, 1 equiv.) and silver salt AgNTf2 (4.2 mg, 0.011 mmol, 2.1 equiv.)were suspended in anhydrous dichloromethane (1 mL), in a vial covered in aluminum foil. The reaction mixturewas stirred at room temperature for 15 minutes. The mixture was filtered off through a pad of Celite to removethe AgCl precipitated, and transferred to an oven dried pressure tube. The solvent was evaporated under vacuumand the active catalyst species used in-situ.The pressure tube impregnated with the active iridium species Ir-3- NTf2 (0.005 mmol, 2 mol%) was loadedwith dry TFE (1 mL). The corresponding amino acid (0.25 mmol, 1 equiv.) and alcohol (0.37 mmol, 1.5 equiv.)were added and the mixture was stirred at 90 °C for 20 hours. Then, the mixture is cooled to 50 °C and uponaddition of methanol (0.1 mL), it is left stirring for 10 h. The crude was then cooled down to r.t., and underhydrogen atmosphere, palladium on carbon (10 wt%, 1 mol%) was added to the mixture. After 5 hours, the crudeis filtered through a pad of Celite, dried under vacuum, and washed with diethyl ether (3x3 mL) to remove theexcess of alcohol and the catalyst. Excellent isolated yields were obtained in all substrates without the need ofany further workup or purification (Scheme S7).
References:
Bermejo-López, Aitor;Raeder, Majken;Martínez-Castro, Elisa;Martín-Matute, Belén [Chem,2022,vol. 8,# 12,p. 3302 - 3323] Location in patent:supporting information
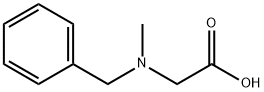
5616-81-9
145 suppliers
$12.00/1g

107-97-1
600 suppliers
$5.00/100mg
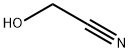
107-16-4
0 suppliers
$134.00/10ml

74-89-5
0 suppliers
$13.44/25ML

107-97-1
600 suppliers
$5.00/100mg
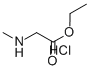
52605-49-9
357 suppliers
$6.00/10g

107-97-1
600 suppliers
$5.00/100mg

50-00-0
892 suppliers
$10.00/25g
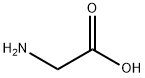
56-40-6
1338 suppliers
$5.00/25g

107-97-1
600 suppliers
$5.00/100mg