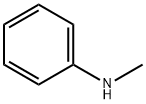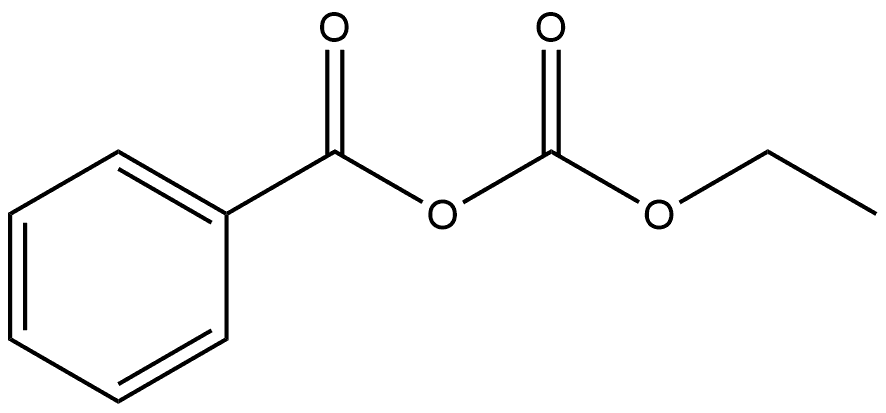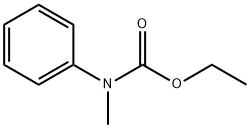
N-methyl-N-phenylurethane synthesis
- Product Name:N-methyl-N-phenylurethane
- CAS Number:2621-79-6
- Molecular formula:C10H13NO2
- Molecular Weight:179.22
Yield: 88%
Reaction Conditions:
Stage #1:N-methylaniline with triethylamine in dichloromethane for 0.333333 h;
Stage #2:chloroformic acid ethyl ester at 20 - 25;
Steps:
Intermediate S2-A Ethyl methyl (phenyl) carbamate
[00186] A solution of N-methylaniline (30 g, 0.28 mol) in DOM (100 mL) was treatedwith triethylamine (45 mL, 0.33 mol) and stirred for 20 mi Ethyl chloroformate (32 mL, 0.33mol) was added over 25 mm with ice bath cooling maintaining the temperature at <25 °0. The reaction mixture was stirred at room temperature overnight, then poured into water (400 mL), acidified with 2 M HCl and extracted with EtOAc (700 mL). The organic layer was separated and washed with brine (400 mL), dried over Na2SO4 and evaporated to dryness to yield ethyl methyl(phenyl)carbamate (44 g, 0.25 mol, 88%).[00187] 1H NMR (300MHz, ODd3) O = 7.40-7.32 (m, 2H), 7.30-7.19 (m, 3H), 4.19(q, J= 7.1 Hz, 2H), 3.32 (5, 3H), 1.25 (t, J= 7.1 Hz, 3H)
References:
CANCER RESEARCH TECHNOLOGY LIMITED;MCGONAGLE, Alison E.;JORDAN, Allan M.;WASZKOWYCZ, Bohdan;HUTTON, Colin P.;WADDELL, Ian D.;HITCHIN, James R.;SMITH, Kate M.;HAMILTON, Niall M. WO2016/97749, 2016, A1 Location in patent:Paragraph 00186; 00187
33224-04-3
0 suppliers
inquiry
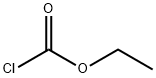
541-41-3
0 suppliers
$19.39/5g

2621-79-6
48 suppliers
$33.39/250mg
7669-54-7
161 suppliers
$51.89/10g
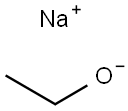
141-52-6
506 suppliers
$10.00/5g

132431-15-3
0 suppliers
inquiry

2621-79-6
48 suppliers
$33.39/250mg
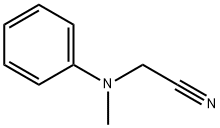
36602-08-1
10 suppliers
inquiry

2621-79-6
48 suppliers
$33.39/250mg